Search Results
Results for: 'hydrochloric acid'
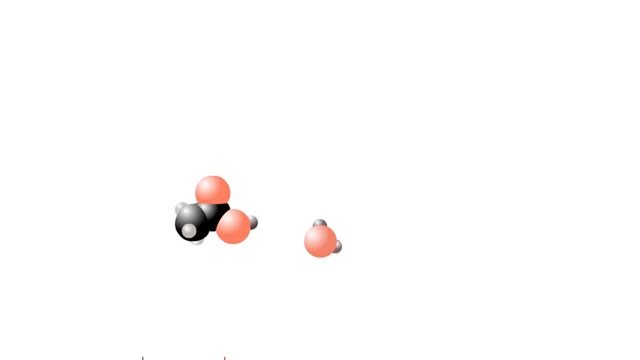
What are Strong & Weak Acids and How they're different?
By: HWC, Views: 8448
Let's consider the changes that take place when hydrogen chloride, HCI, is added to water. You will need to recognize space-filling models of HCI molecules, hydronium ions (H30+), chloride ions (C11, and water molecules (H20). They are shown at the right. When HC1 molecules dissolve in water, ...
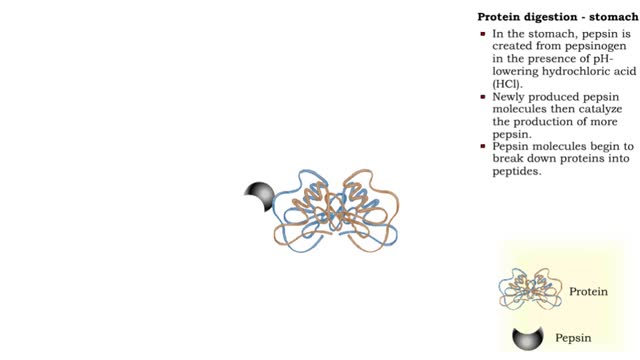
Protein digestion - stomach & small intestine
By: HWC, Views: 9082
• Protein digestion occurs in the stomach and small intestine. • The stomach enzyme pepsin initiates the process. • Pancreatic and intestinal brush border enzymes complete the digestive process. • In the stomach, pepsin is created from pepsinogen in the presence of pH-lowering hyd...
Digestive chemicals - water, gastric acid, bile & bicarbonate
By: HWC, Views: 9402
• Water is the most abundant molecule in ingested fluids. • Water plays a primary role in hydrolytic digestive reactions. • Helps liquefy and transport digestive foodstuffs down the tract. • Transports secretions from accessory digestive organs to gastrointestinal tract. • Aids ...
By: HWC, Views: 9732
The endocrine system maintains many body conditions within normal limits with feedback loops. Each endocrine feedback loop maintains homeostasis using the following components: • Stimulus - a change in a body condition. • Production cell - an endocrine cell that produces a hormone after b...
By: HWC, Views: 9420
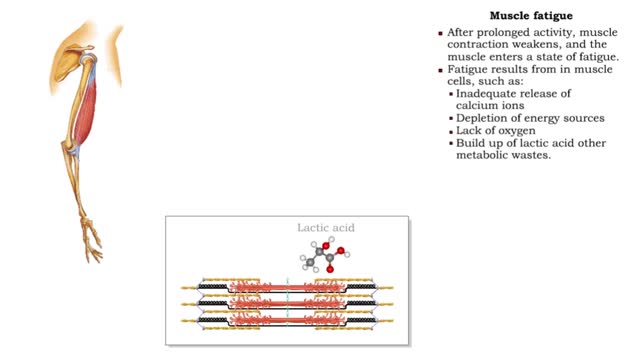
• After prolonged activity, muscle contraction weakens, and the muscle enters a state of fatigue. • Fatigue results from in muscle cells, such as: • Inadequate release of calcium ions • Depletion of energy sources • Lack of oxygen • Build up of lactic acid other metabolic w...
Major Elements in Biological Molecules: Lipids
By: HWC, Views: 9022
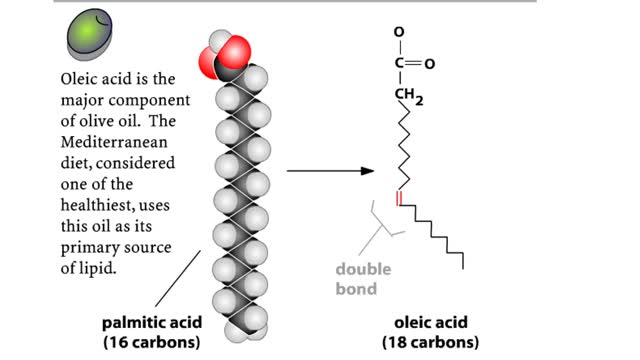
A triglyceride (also called triacylglycerol) is composed of three fatty acid molecules and one glycerol molecule. The fatty acids attach to the glycerol molecule by a covalent ester bond. The long hydrocarbon chain of each fatty acid makes the triglyceride molecule nonpolar and hydrophobic. Pa...
Buffers definition and the role of buffer in the body
By: HWC, Views: 9774
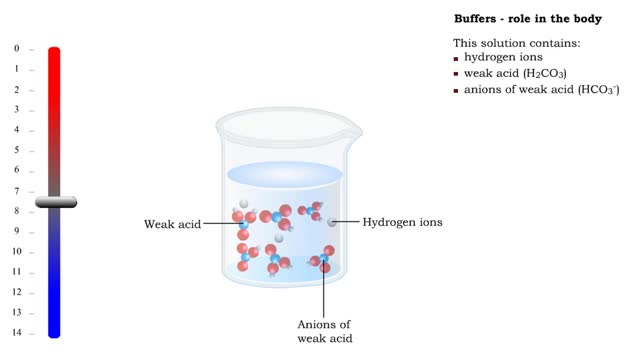
■ Too many H+ break hydrogen bonds and a protein comes apart. ■ Buffers react with excess H+ to protect proteins from breaking down. ■ Buffers consist of weak acid plus anions of that weak acid. This solution contains: • hydrogen ions • weak acid (H2CO3) • anions of we...
By: HWC, Views: 9981
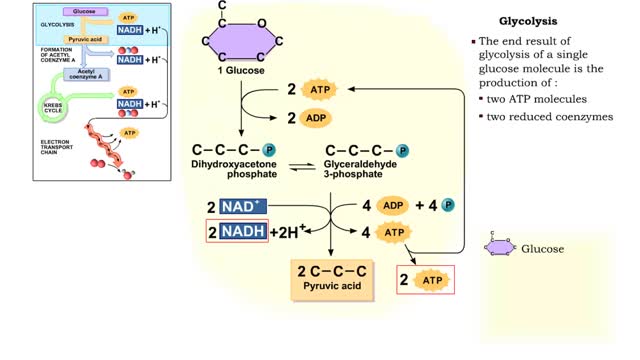
The first reactions involve a single 6-carbon glucose sugar undergoing phosphorylation using two ATP molecules and resulting in two 3-carbon compounds. • The rest of this pathway involves an oxidation reduction reaction, forming two reduced coenzymes, and generation of four ATP molecules. ...
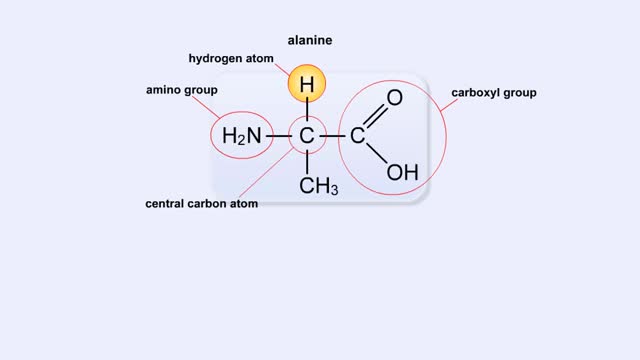
Structure of Amino Acid, Peptide Bonds & Polypeptides
By: HWC, Views: 9204
Here are the molecular formulas of three different amino acids. All amino acids share this backbone. The main difference between every amino acid is the side groups seen here, and these side groups give each of the amino acids their different characteristics. But before we get into that, let's ...
Advertisement