Search Results
Results for: 'kinetic energy'
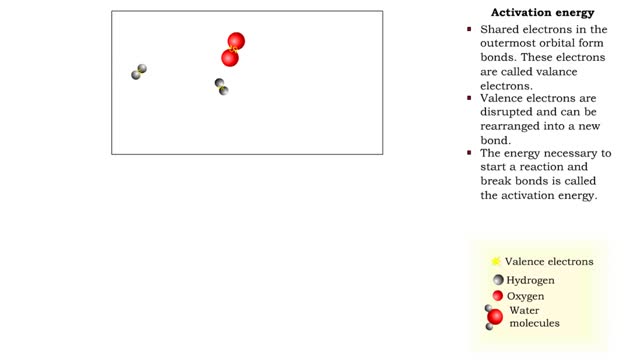
Activation Energy - Valence Electrons
By: HWC, Views: 10780
■ Shared electrons in the outermost orbital form bonds. These electrons are called valence electrons. ■ Valence electrons are disrupted and can be rearranged into a new bond. ■ The energy necessary to start a reaction and break bonds is called the activation energy. ■ Reactants have...
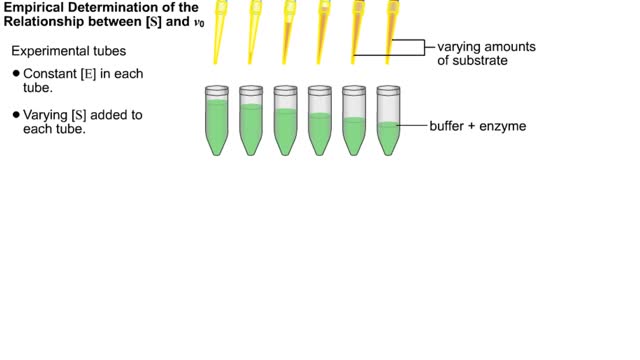
Kinetic parameters & Kinetic experiment
By: HWC, Views: 11162
Kinetics is a measure of the speed or rate of a chemical reaction. A study of kinetics allows us to determine which variables to control (temperature, reactants, catalysts) and how to vary them in order to maximize the amount of products formed and minimize the time involved. Vmax = maximum ve...
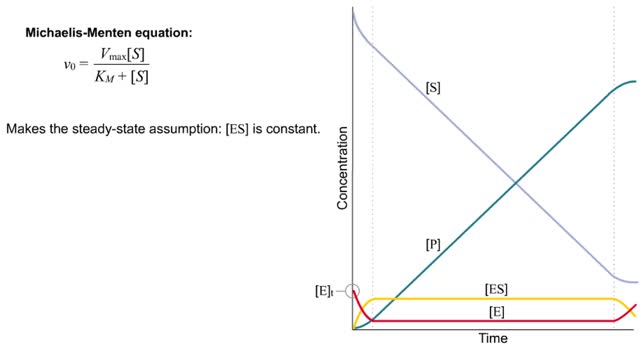
Michaelis–Menten equation & Kinetic parameters
By: HWC, Views: 11159
The Michaelis–Menten equation is the rate equation for a one-substrate enzyme-catalyzed reaction. This equation relates the initial reaction rate (v0), the maximum reaction rate (Vmax), and the initial substrate concentration [S] through the Michaelis constant KM—a measure of the substrat...
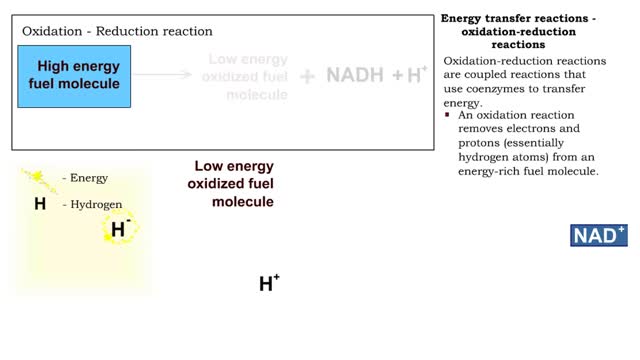
Types of energy transfer reactions: oxidation-reduction reactions and ATP generation reactions
By: HWC, Views: 11950
■ Metabolism balances anabolic and catabolic reactions. ■ Anabolism is energy transfer from ATP to simpler molecules in order to build them up into larger, more complex molecules. ■ Catabolism is breaking down larger, more complex molecules, usually to transfer energy from them in order...
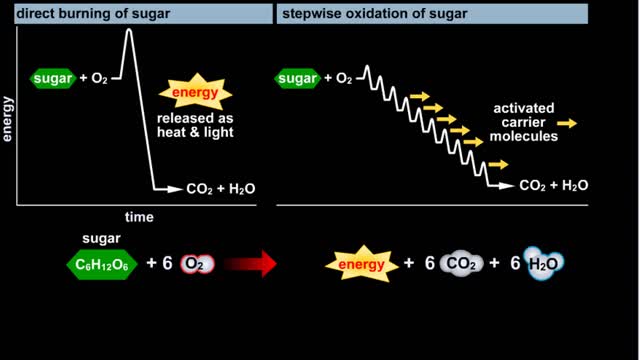
Glycolysis - Introduction to ATP and the burning of sugar
By: HWC, Views: 11555
Do you use sugar with your coffee or tea? Or do you occasionally drink a sport or soft drink? As millions of people do each day, they obtain energy from the sugar added or contained in these drinks. How can we understand this concept of energy within a sugar molecule? Let's take a tablespoon ...
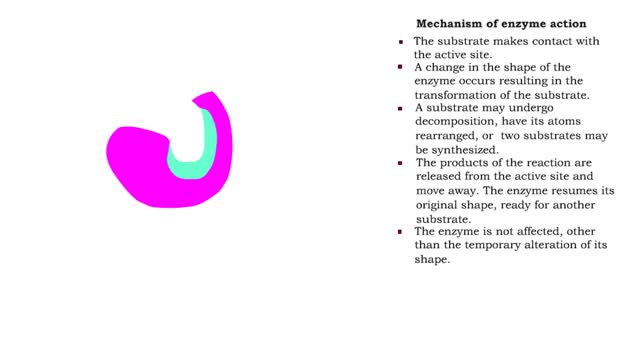
By: HWC, Views: 11160
■ The substrate makes contact with the active site. ■ A change in the shape of the enzyme occurs resulting in the transformation of the substrate. ■ A substrate may undergo decomposition, have its atoms rearranged, or two substrates may be synthesized. ■ The products of the reaction...
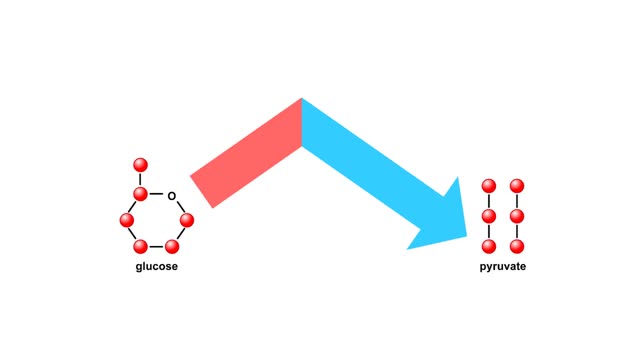
Energy inputs and release in glycolysis Animation
By: HWC, Views: 5183
Glycolysis breaks the six-carbon sugar glucose into two three-carbon molecules of pyruvate. The first steps of glycolysis require an energy input in the form of two phosphate-group transfers from ATP. These phosphorylations raise the energy level of glucose enough to allow the energy-releas...
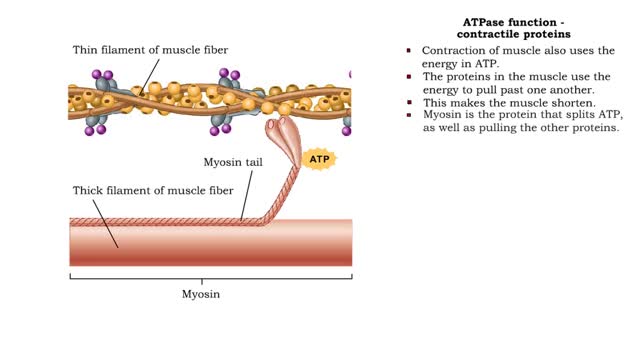
ATPase function - membrane transport, contractile proteins and synthesis
By: HWC, Views: 11760
• Energy from ATP is used to move ions across the cell membrane during active transport. • This membrane protein transports sodium out of the cell and potassium into the cell. As such, it is called a sodium-potassium pump. • Because this pump also acts as an enzyme to hydrolyze ATP it i...
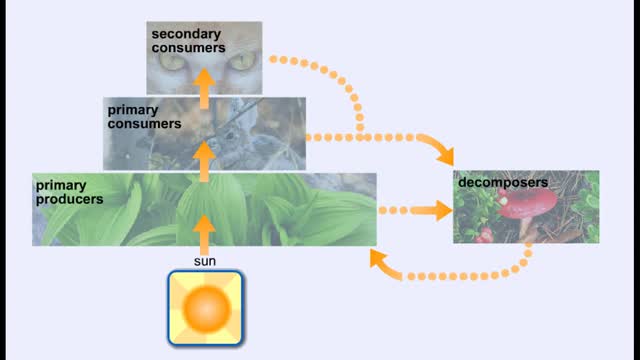
Energy Flow - Trophic Levels and Food
By: HWC, Views: 10927
All of these relationships between different species are founded on one thing: energy. Organisms get food in order to get energy, which is used by the organism for growth, maintaining health, and reproduction. We can classify the members of a community according to how they obtain food. Produc...
Advertisement