Search Results
Results for: 'pH'
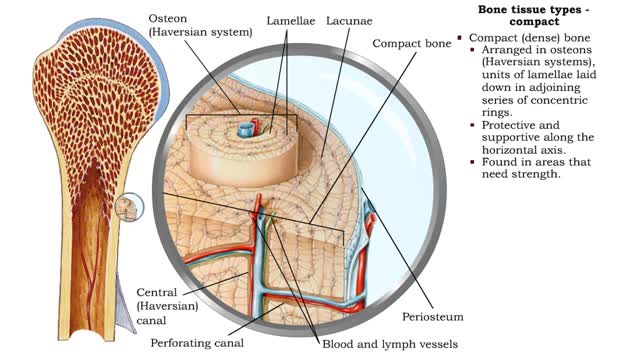
Bone tissue types - compact and spongy
By: HWC, Views: 11015
Bone tissue types • There are two types of bone tissue: compact and spongy. • All the bones of the skeleton have both kinds of bone tissue. • Compact (dense) bone • Arranged in osteons (Haversian systems), units of lamellae laid down in adjoining series of concentric rings. • P...
By: HWC, Views: 11365
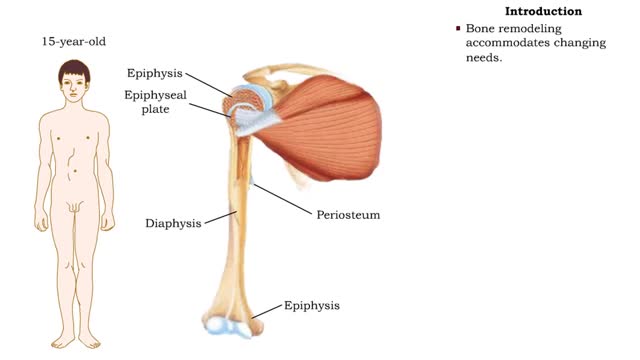
• After birth, bones grow in thickness and length. • Bones grow in diameter via appositional growth at the periosteum. • Epiphyseal plates enable lengthwise growth of long bones, such as the humerus, by interstitial growth. • Bone remodeling accommodates changing needs. • While th...
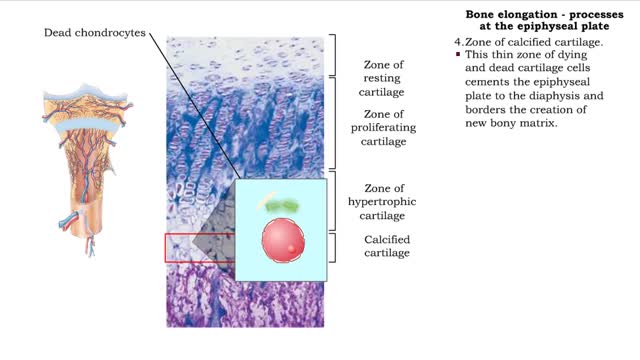
Bone elongation - processes at the epiphyseal plate
By: HWC, Views: 11180
• Interstitial lengthening occurs in only certain bones, primarily those of the appendages. • Such lengthening takes place at the epiphyseal plate, a layer of hyaline cartilage in the metaphysis of a growing bone. 1. Zone of resting cartilage. • Consisting of a hyaline cartilage pa...
By: HWC, Views: 11098
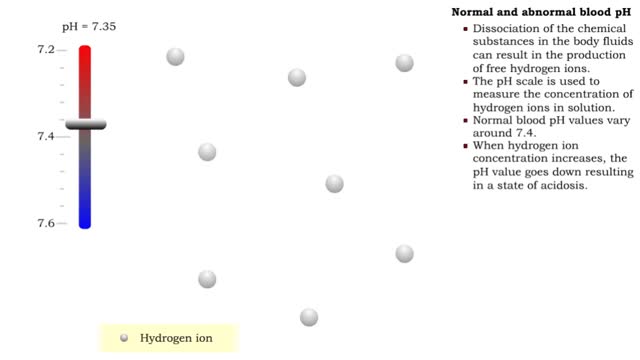
• Dissociation of the chemical substances in the body fluids can result in the production of free hydrogen ions. • The pH scale is used to measure the concentration of hydrogen ions in solution. • Normal blood pH values vary around 7.4. • When hydrogen ion concentration increases, t...
By: HWC, Views: 11151
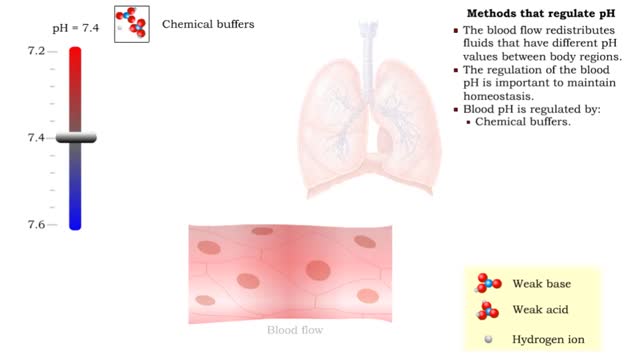
• The blood flow redistributes fluids that have different pH values between body regions. • The regulation of the blood pH is important to maintain homeostasis. • Blood pH is regulated by: • Chemical buffers. • The respiratory system. • The urinary system. • All thes...
Chemical Buffers - protein buffer, phosphate buffer system and bicarbonate buffer system
By: HWC, Views: 11206
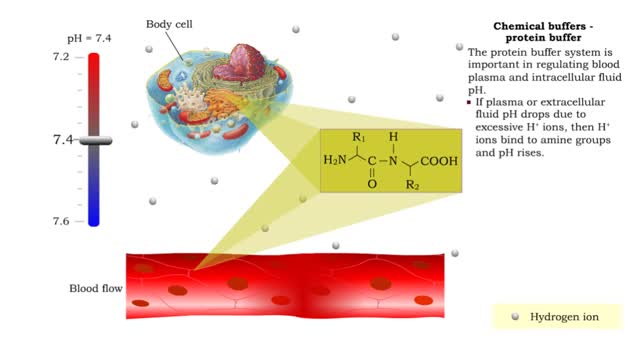
• There are a variety of chemicals in body fluids that prevent the fluids from undergoing large changes in. • These chemicals buffer or regulate fluctuations in H+ concentration. • Chemical buffers: • Bind to H+ ions when there are too many in a solution so pH remains normal. •...
Role of the respiratory system - effect of altered ventilation rates
By: HWC, Views: 11626
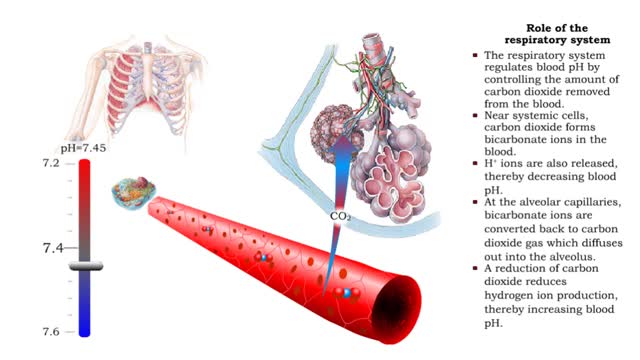
• The respiratory system regulates blood pH by controlling the amount of carbon dioxide removed from the blood. • Near systemic cells, carbon dioxide forms bicarbonate ions in the blood. H+ ions are also released, thereby decreasing blood pH. • At the alveolar capillaries, bicarbonate io...
Role of the urinary system - acidosis and alkalosis
By: HWC, Views: 11172
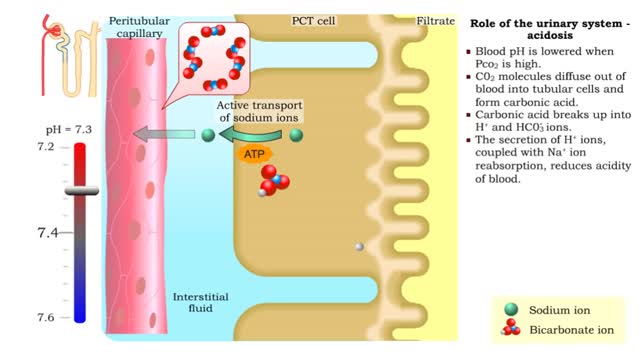
• Tubular cells of the proximal convoluted tubule and collecting tubules can alter filtrate pH and therefore blood pH. • These cells can affect blood pH with two coupled mechanisms: • Reabsorption of bicarbonate ions. • Secretion of hydrogen ions. • The reabsorption of bicarbonate...
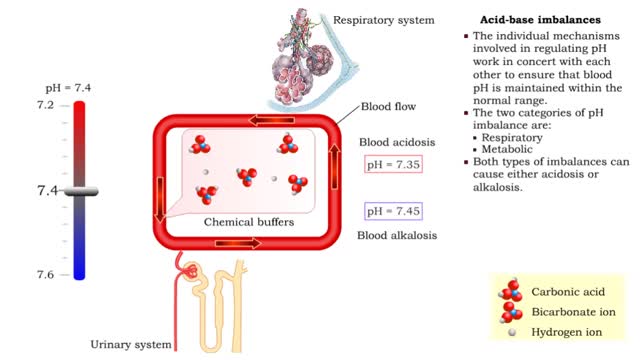
Acid-base imbalances - respiratory acidosis and alkalosis
By: HWC, Views: 11364
• The individual mechanisms involved in regulating pH work in concert with each other to ensure that blood pH is maintained within the normal range. • The two categories of pH imbalance are: • Respiratory • Metabolic • Both types of imbalances can cause either acidosis or alka...
Advertisement