Search Results
Results for: 'pH'
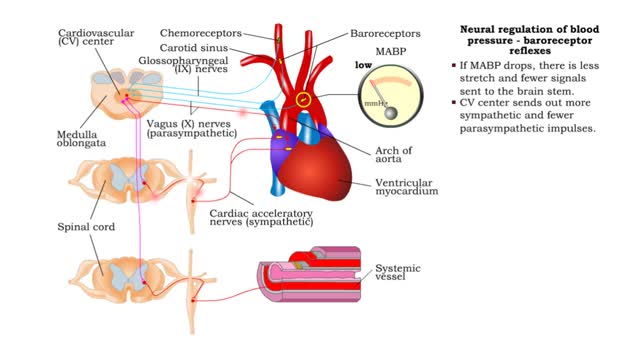
Neural regulation of blood pressure - baroreceptor and chemoreceptor reflexes
By: HWC, Views: 11441
• The nervous system regulates blood pressure with two reflex arcs: baroreceptor and chemoreceptor. ■ Baroreceptors (pressure) and chemoreceptors (chemical) are located in the carotid sinus and aortic arch. • Carotid sinus reflex helps maintain normal blood pressure in brain. • Ba...
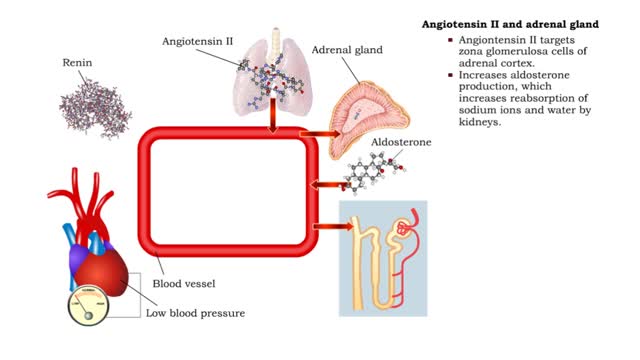
Angiotensin II - kidneys, adrenal glands and dehydration
By: HWC, Views: 11146
• Angiontensin II targets cells in the proximal convoluted tubule of the nephron. ■ The reabsorption of Na+ and Cl- ions sets up an osmotic gradient favoring the retention of water. • Decreases urine production and increases blood volume and pressure. • Angiontensin II targets zon...
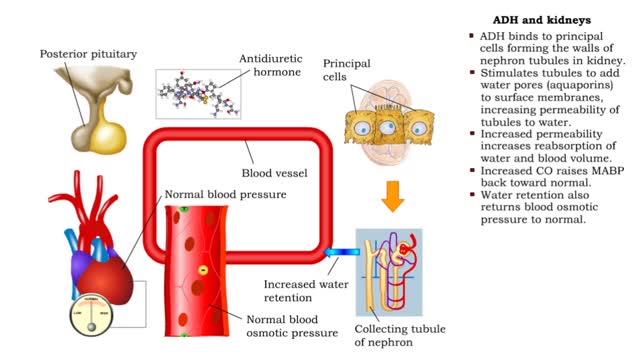
ADH and the arterioles, kidneys, sweat glands and the Atrial natriuretic peptide (ANP)
By: HWC, Views: 11170
• ADH is also known as vasopressin. • Produced by hypothalmus and secreted by neurosecretory cells in posterior pituitary gland. • Responds to high blood osmotic pressure representing low amounts of water in the blood. • Binds to smooth muscle cells in walls of arterioles, stimulate...
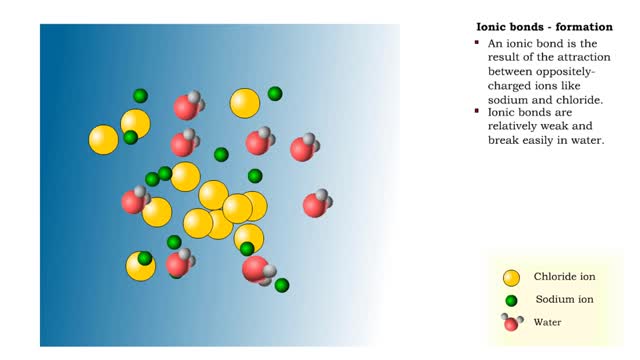
Ionic bonds - role of ions in the body
By: HWC, Views: 11344
Ions • Atoms fill up the outer orbital by transferring electrons from one atom to another. • Atoms now bear a charge and are called ions. • Sodium ion, losing an electron, has a +1 charge. • Chlorine ion, gaining an electron, has a -1 charge. Formation • An ionic bond is t...
Non-polar compounds - insolubility
By: HWC, Views: 11192
• A non-polar molecule has uniform distribution of electrons. • Non-polar compounds like fatty acids in lipids have a high proportion of carbon and hydrogen. • Lipids possess no charge or partial charge. • Lipids are not attracted to water molecules. • Lipids are not soluble in...
The pH scale - Strong acids and Weak acids
By: HWC, Views: 11150
The pH scale • Expresses concentration of H+. • range: 0-14. • 7 is neutral. • Less 7 is acid. • greater 7 is basic (alkaline). Strong acids - role in the body ■ In strong acids all molecules dissociate. ■ HC1 is highly acidic and found only in the stomach. • H...
Buffers definition and the role of buffer in the body
By: HWC, Views: 11105
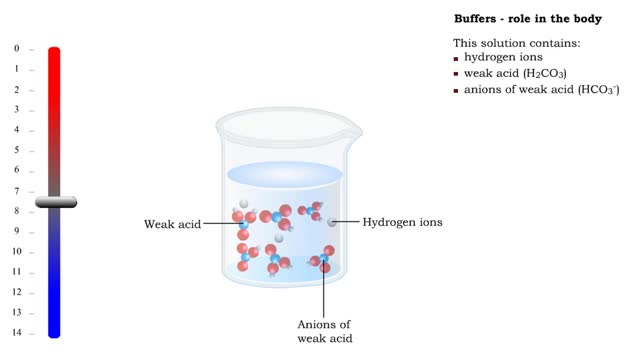
■ Too many H+ break hydrogen bonds and a protein comes apart. ■ Buffers react with excess H+ to protect proteins from breaking down. ■ Buffers consist of weak acid plus anions of that weak acid. This solution contains: • hydrogen ions • weak acid (H2CO3) • anions of we...
By: HWC, Views: 10881
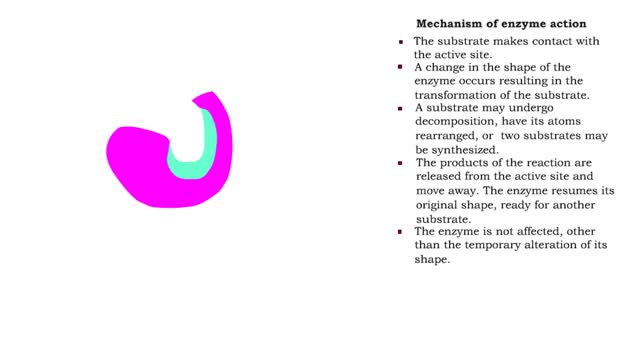
■ The substrate makes contact with the active site. ■ A change in the shape of the enzyme occurs resulting in the transformation of the substrate. ■ A substrate may undergo decomposition, have its atoms rearranged, or two substrates may be synthesized. ■ The products of the reaction...
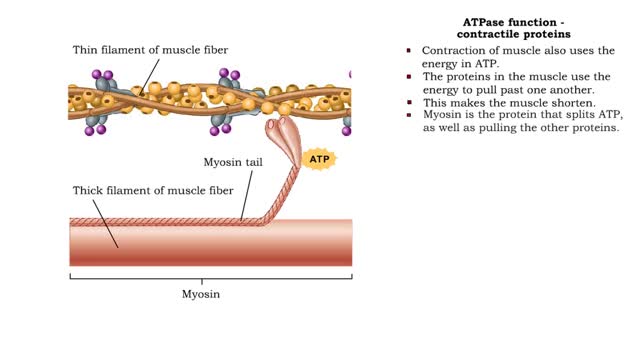
ATPase function - membrane transport, contractile proteins and synthesis
By: HWC, Views: 11500
• Energy from ATP is used to move ions across the cell membrane during active transport. • This membrane protein transports sodium out of the cell and potassium into the cell. As such, it is called a sodium-potassium pump. • Because this pump also acts as an enzyme to hydrolyze ATP it i...
Advertisement