Search Results
Results for: 'Hydrogen bonds vs. Ionic bond'
By: HWC, Views: 5291
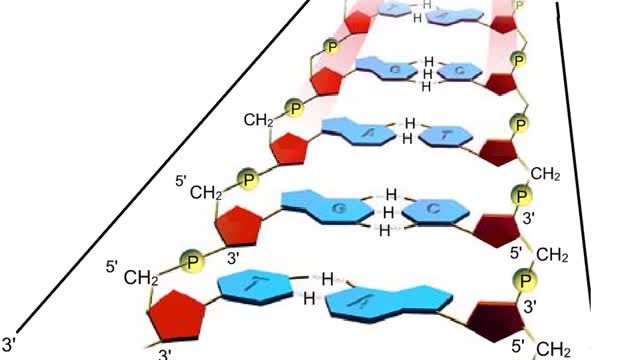
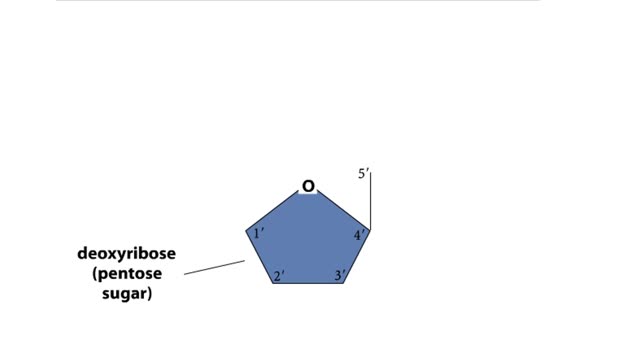
A section from a DNA double helix The backbone of each DNA strand consists of alternating deoxyribose sugars and phosphate groups. The two strands run in opposite directions. One runs from the 5' to 3' direction, the other in the 3' to 5' direction. Think of the deoxyribose units o...
Major Elements in Biological Molecules: Nucleic acids
By: HWC, Views: 11324
DNA and RNA are nucleic acids (polymers of nucleotides). Two polymers with complementary nucleotide sequences can pair with each other. This pairing endows nucleic acids with the ability to store, transmit, and retrieve genetic information. Two strands of DNA pair by hydrogen bonding. A compon...
By: HWC, Views: 11168
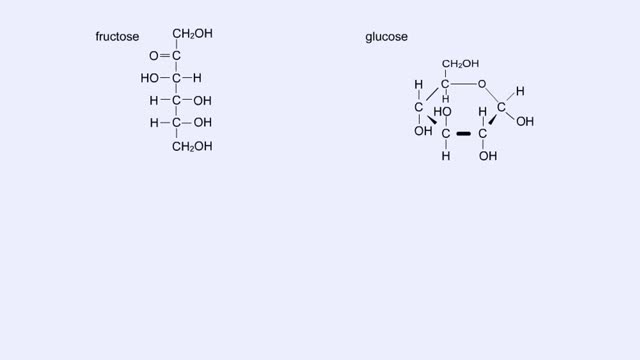
Here are the molecular structures of three simple sugars: glucose, ribose, and fructose. Look at these simple sugars and identify what characteristics they all share. As you can see, all of the carbohydrates have carbon, hydrogen, and oxygen in a ratio of 1:2:1 and there is always a double bo...
By: HWC, Views: 10894
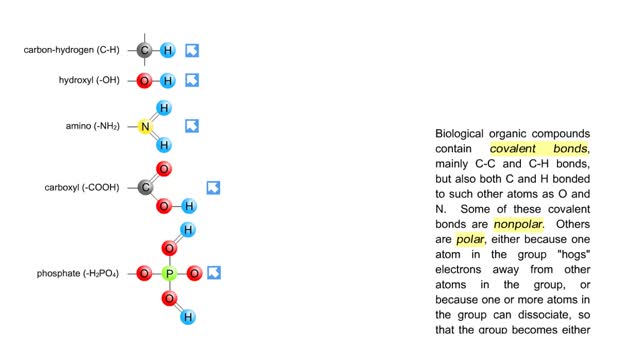
Biological organic compounds contain covalent bonds, mainly C-C and C-H bonds, but also both C and H bonded to such other atoms as O and N. Some of these covalent bonds are nonpolar. Others are polar, either because one atom in the group "hogs" electrons away from other atoms in the group, or...
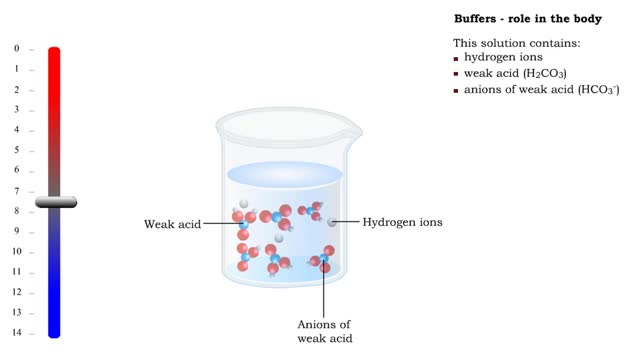
Buffers definition and the role of buffer in the body
By: HWC, Views: 11431
■ Too many H+ break hydrogen bonds and a protein comes apart. ■ Buffers react with excess H+ to protect proteins from breaking down. ■ Buffers consist of weak acid plus anions of that weak acid. This solution contains: • hydrogen ions • weak acid (H2CO3) • anions of we...
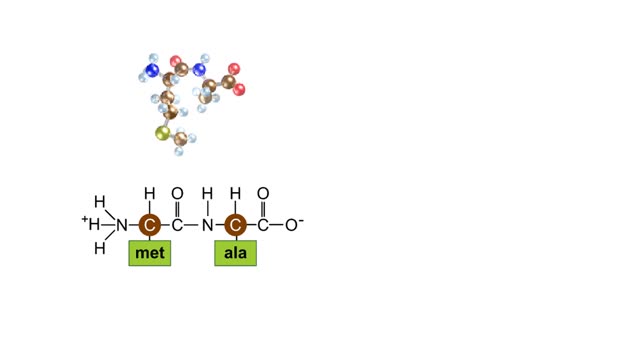
Peptide Bond Formation Animation
By: HWC, Views: 5205
During protein synthesis, peptide bonds link amino acids together in the order specified by DNA instructions. In this case, the first two amino acids in the protein are methionine and alanine. Here are ball-and-stick models of these amino acids. Peptide bond formation is a type of condensatio...
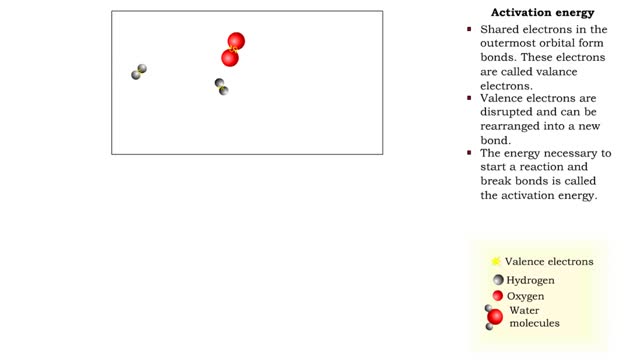
Activation Energy - Valence Electrons
By: HWC, Views: 10852
■ Shared electrons in the outermost orbital form bonds. These electrons are called valence electrons. ■ Valence electrons are disrupted and can be rearranged into a new bond. ■ The energy necessary to start a reaction and break bonds is called the activation energy. ■ Reactants have...
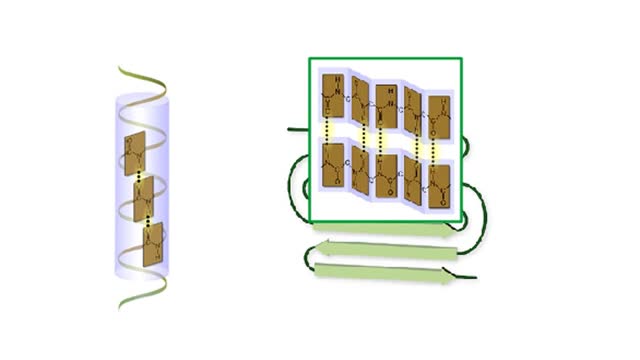
Protein Secondary and Tertiary Structures - Animation
By: HWC, Views: 7597
Amino acid sequence dictates a protein's final shape. The presence of certain amino acids favors a pattern of hydrogen bonding that causes part of the polypeptide chain to coil and twist into an alpha helix. The presence of other amino acids enables hydrogen bonding between strand like r...
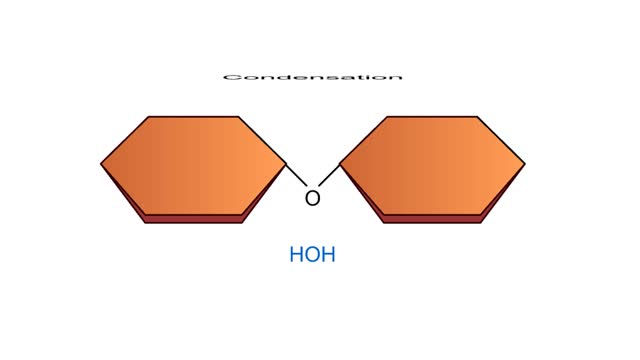
Condensation and Hydrolysis Animation
By: HWC, Views: 5176
A condensation reaction joins two molecules together to form one larger molecule. An enzyme removes a hydroxyl group from one molecule and a hydrogen atom from another, then speeds the formation of a bond between the two molecules at their exposed sites. Typically the discarded atoms join t...
Advertisement