Search Results
Results for: 'Peptide Bond Formation Animation'
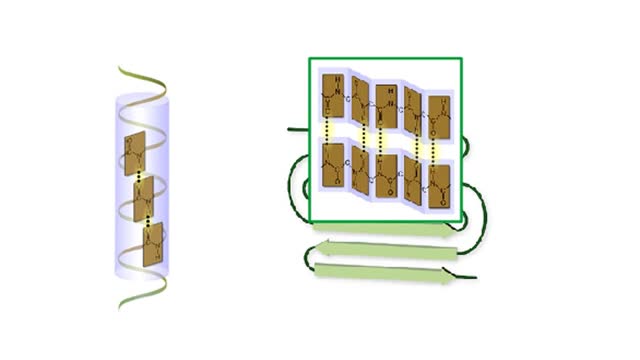
Protein Secondary and Tertiary Structures - Animation
By: HWC, Views: 5836
Amino acid sequence dictates a protein's final shape. The presence of certain amino acids favors a pattern of hydrogen bonding that causes part of the polypeptide chain to coil and twist into an alpha helix. The presence of other amino acids enables hydrogen bonding between strand like r...
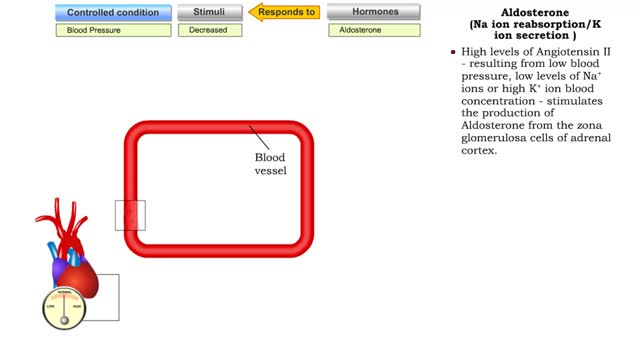
Atrial natriuretic peptide (vasodilation) & Aldosterone
By: HWC, Views: 9411
• Certain situations will cause the body's stress level to rise. • increased blood pressure will stretch the atria of the heart, stimulating the secretion of atria natriuretic peptide (MP). • ANP causes muscle cells in blood vessels to relax. • Blood pressure is lowered as a result ...
Bond in biological molecules (Ionic, Covalent and Hydrogen bonds)/ How atoms bond?
By: HWC, Views: 7030
Sodium atoms and chloride atoms have unfilled orbitals in their outer shells. The lone electron in the outermost shell of a sodium atom can be pulled or knocked out. This ionizes the atom. It is now a positively charged sodium ion. A chlorine atom has an electron vacancy in its outer shell and...
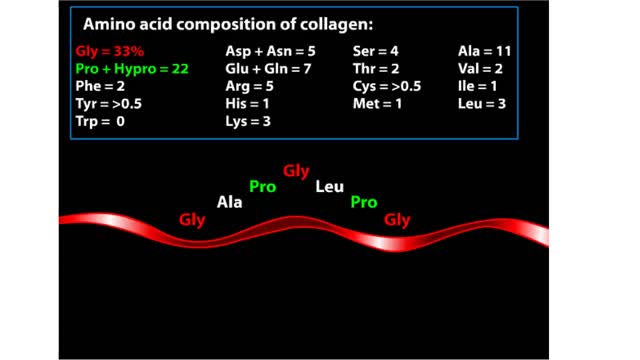
Major Elements in Biological Molecules: Proteins
By: HWC, Views: 9184
Proteins are chains of amino acids linked by peptide bonds. The 20 different amino acids used to make all proteins differ only in their side chains, and the properties of these side chains account for the great diversity of protein structure and function. Collagen is an example of how a prote...
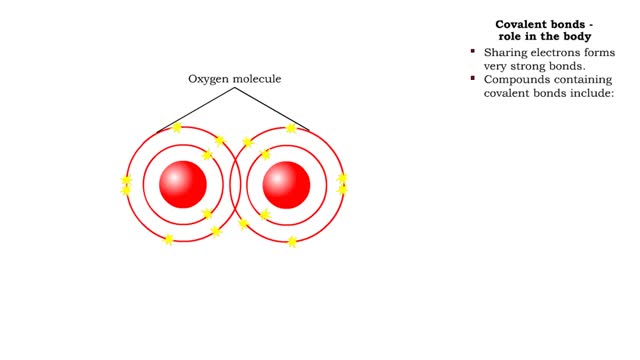
Covalent bonds - role in the body
By: HWC, Views: 9661
A covalent bond is formed when atoms share one or more pairs of electrons. This is opposed to an ionic bond, where electrons are actually transferred from one atom to another. Formation • Atoms fill up the outer orbital by sharing electrons. • Two oxygen atoms sharing electrons form on...
Hydrogen bonds - role in the body
By: HWC, Views: 9988
A hydrogen bond is the electromagnetic attraction between polar molecules in which hydrogen is bound to a larger atom, such as oxygen or nitrogen. This is not a sharing of electrons, as in a covalent bond. Instead, this is an attraction between the positive and negative poles of charged atoms. ...
By: HWC, Views: 9469
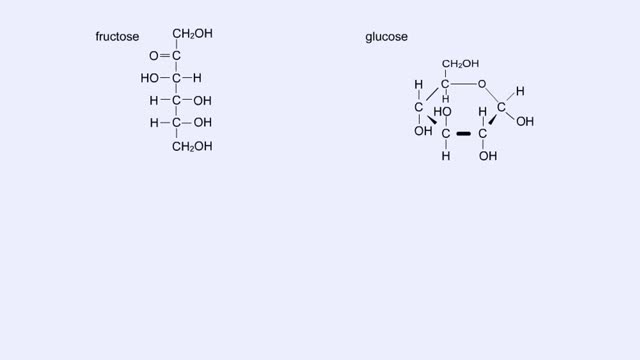
Here are the molecular structures of three simple sugars: glucose, ribose, and fructose. Look at these simple sugars and identify what characteristics they all share. As you can see, all of the carbohydrates have carbon, hydrogen, and oxygen in a ratio of 1:2:1 and there is always a double bo...
Major Elements in Biological Molecules: Lipids
By: HWC, Views: 9061
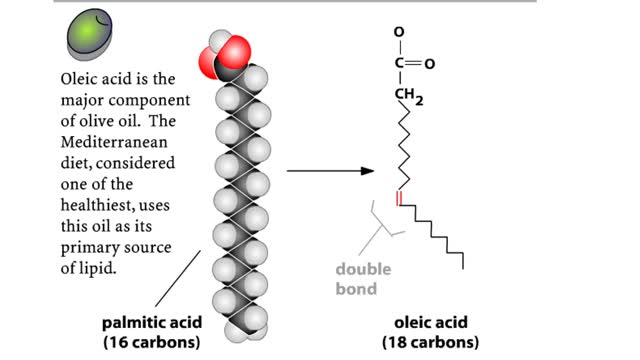
A triglyceride (also called triacylglycerol) is composed of three fatty acid molecules and one glycerol molecule. The fatty acids attach to the glycerol molecule by a covalent ester bond. The long hydrocarbon chain of each fatty acid makes the triglyceride molecule nonpolar and hydrophobic. Pa...
By: HWC, Views: 9504
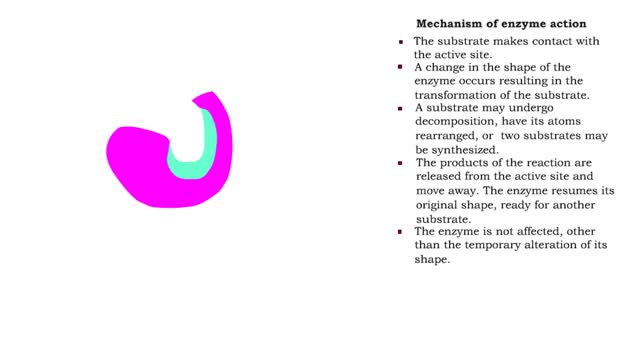
■ The substrate makes contact with the active site. ■ A change in the shape of the enzyme occurs resulting in the transformation of the substrate. ■ A substrate may undergo decomposition, have its atoms rearranged, or two substrates may be synthesized. ■ The products of the reaction...
Advertisement