Search Results
Results for: 'ED'
ADH and the arterioles, kidneys, sweat glands and the Atrial natriuretic peptide (ANP)
By: HWC, Views: 11036
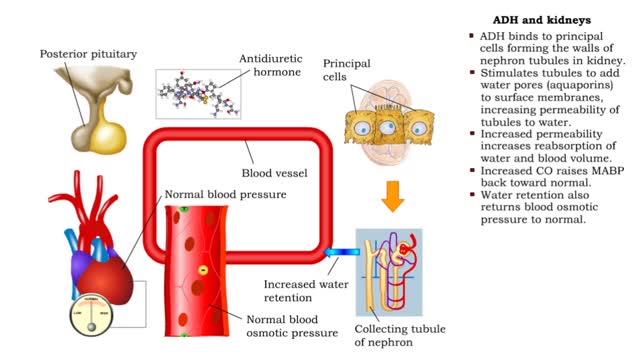
• ADH is also known as vasopressin. • Produced by hypothalmus and secreted by neurosecretory cells in posterior pituitary gland. • Responds to high blood osmotic pressure representing low amounts of water in the blood. • Binds to smooth muscle cells in walls of arterioles, stimulate...
Negative Feedback Regulation of Blood Pressure
By: HWC, Views: 10919
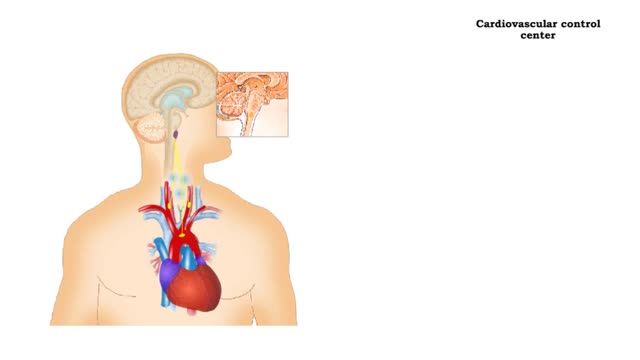
stimulus • Blood pressure determines the flow of blood to and from capillaries. • Low blood pressure results in reduced blood flow. • High blood pressure can cause blood vessels to break. Baroreceptors • The aortic arch carries blood to the body. • The common carotids ca...
Bond types - Atomic structure and basis of bonds
By: HWC, Views: 11339
• Chemical bonds are fundamental to the structure and function of many types of molecules, such as proteins, carbohydrates, lipids, nucleic acids, gases, salts and water. ■ These molecules are composed of atoms that are held together by three different types of bonds. • The three types ...
Ionic bonds - role of ions in the body
By: HWC, Views: 11210
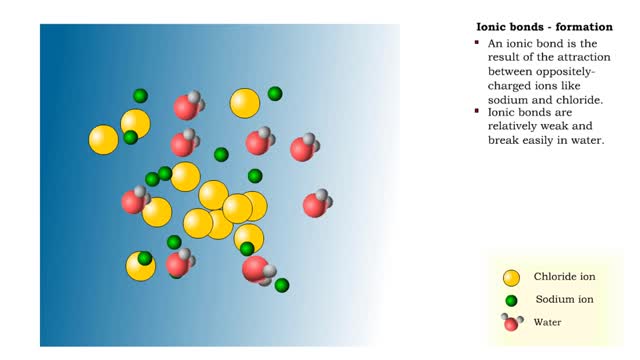
Ions • Atoms fill up the outer orbital by transferring electrons from one atom to another. • Atoms now bear a charge and are called ions. • Sodium ion, losing an electron, has a +1 charge. • Chlorine ion, gaining an electron, has a -1 charge. Formation • An ionic bond is t...
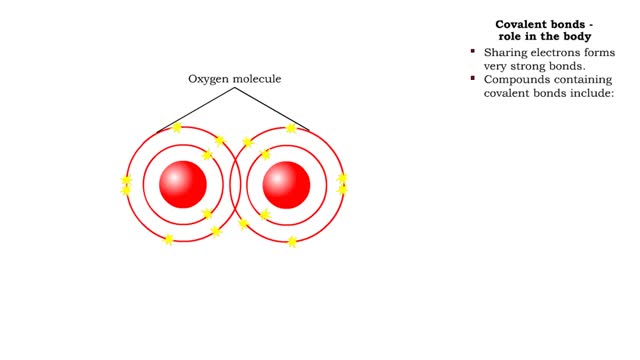
Covalent bonds - role in the body
By: HWC, Views: 10898
A covalent bond is formed when atoms share one or more pairs of electrons. This is opposed to an ionic bond, where electrons are actually transferred from one atom to another. Formation • Atoms fill up the outer orbital by sharing electrons. • Two oxygen atoms sharing electrons form on...
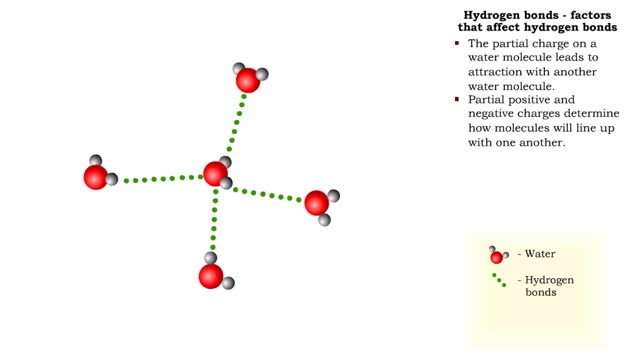
Hydrogen bonds - role in the body
By: HWC, Views: 11249
A hydrogen bond is the electromagnetic attraction between polar molecules in which hydrogen is bound to a larger atom, such as oxygen or nitrogen. This is not a sharing of electrons, as in a covalent bond. Instead, this is an attraction between the positive and negative poles of charged atoms. ...
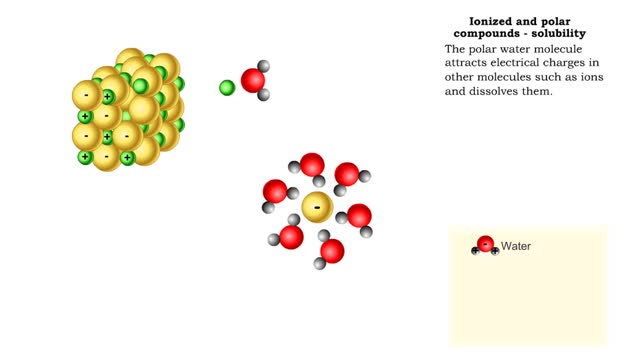
Properties of water -structure of water and polarity (Ionized and polar compounds)
By: HWC, Views: 11075
■ Water transports most of the molecules in the body. ■ The structure of a water molecule allows it to dissolve other molecules. ■ Shared electrons spend more time near the oxygen atom. ■ Oxygen end has a partial negative charge. ■ Hydrogen ends have a partial positive charge....
Non-polar compounds - insolubility
By: HWC, Views: 11057
• A non-polar molecule has uniform distribution of electrons. • Non-polar compounds like fatty acids in lipids have a high proportion of carbon and hydrogen. • Lipids possess no charge or partial charge. • Lipids are not attracted to water molecules. • Lipids are not soluble in...
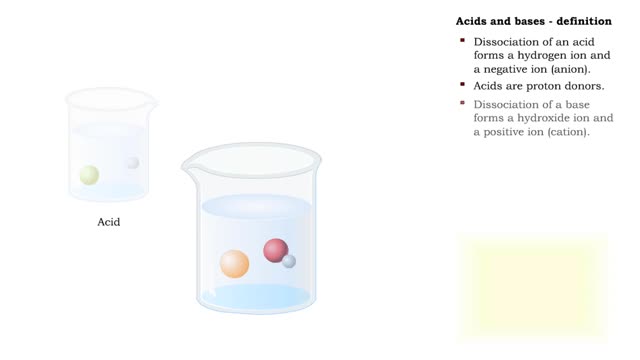
By: HWC, Views: 10830
Acids and bases are found all around your house. For example, if you open your pantry or refrigerator, you might see a lot of acids. Fruit juice, soda pop, vinegar, and milk are all examples of acids. The word acid actually comes from a Latin term meaning ''sour.'' Many materials, like sugar for ...
Advertisement