Search Results
Results for: 'water reabsorption'
Non-polar compounds - insolubility
By: HWC, Views: 11138
• A non-polar molecule has uniform distribution of electrons. • Non-polar compounds like fatty acids in lipids have a high proportion of carbon and hydrogen. • Lipids possess no charge or partial charge. • Lipids are not attracted to water molecules. • Lipids are not soluble in...
Properties of water -structure of water and polarity (Ionized and polar compounds)
By: HWC, Views: 11171
■ Water transports most of the molecules in the body. ■ The structure of a water molecule allows it to dissolve other molecules. ■ Shared electrons spend more time near the oxygen atom. ■ Oxygen end has a partial negative charge. ■ Hydrogen ends have a partial positive charge....
Osmosis - Isotonic, Hypotonic, and Hypertonic Solutions
By: HWC, Views: 11124
Isotonic: Equal Water moves in and out of the cell at an equal rate. The cell remains unchanged. Hypotonic: "hypo" hippo Water moves into the cell, making it swell and get fat (like a hippo). Eventually the cell can rupture and burst (aka lyse). Hypertonic: "like a raisin" Water leaves...
By: HWC, Views: 8204
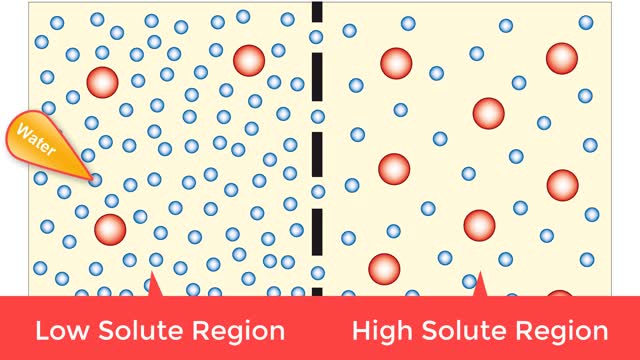
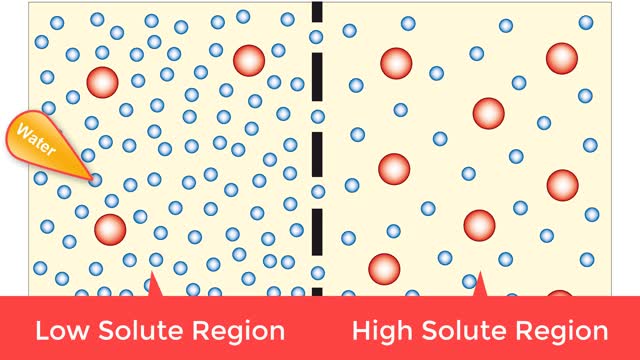
Osmosis is when a solvent, such as water, moves from a low-solute concentration solution to a higher-solute concentration solution through a semipermeable. Osmosis is an example of diffusion (a special case of diffusion) in which the molecules are water, and the concentration gradient occurs a...
By: HWC, Views: 8704
Osmosis is when a solvent, such as water, moves from a low-solute concentration solution to a higher-solute concentration solution through a semipermeable. Osmosis is an example of diffusion (a special case of diffusion) in which the molecules are water, and the concentration gradient occurs a...
What are Strong & Weak Acids and How they're different?
By: HWC, Views: 9805
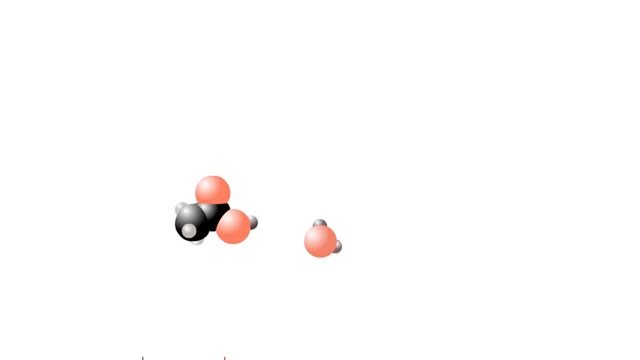
Let's consider the changes that take place when hydrogen chloride, HCI, is added to water. You will need to recognize space-filling models of HCI molecules, hydronium ions (H30+), chloride ions (C11, and water molecules (H20). They are shown at the right. When HC1 molecules dissolve in water, ...
By: HWC, Views: 10308
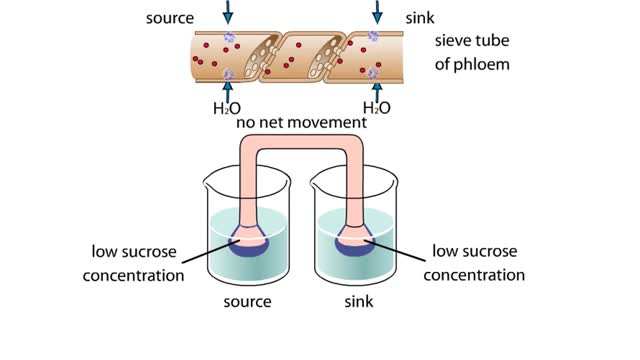
This apparatus of beakers A and funnels simulates the flow of a sucrose solution in the phloem of a plant. The funnels and connecting tube represent a sieve tube of the phloem. Differentially permeable membranes cap the funnels at the source and sink ends, allowing water, but not sucrose, to cros...
Forming urine ( influencing factors), Forming dilute urine & Forming concentrated urine
By: HWC, Views: 11508
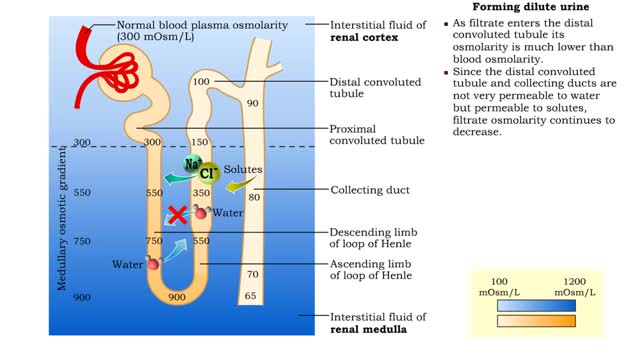
• The amount of urine produced by the nephron depends on : • Body fluid volume. • Body fluid composition. • Dilute urine is formed when the body is normally hydrated. • The medullary osmotic gradient determines the osmolarity of the filtrate. • Filtrate osmolarity increase...
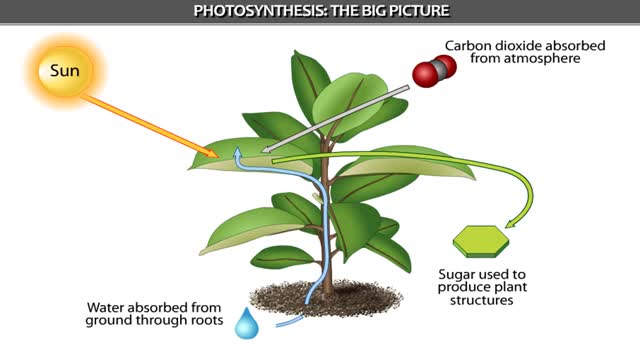
Brief Summary on Photosynthesis - Animation
By: HWC, Views: 10088
Can increase its weight by 150 pounds as it grows. Where does the new tissue come from? From the soil? From water? Or possibly from the air? The amazing truth is that new material. comes from an invisible gas in the air. In the process of photosynthesis, plants capture carbon dioxide ga...
Advertisement