Search Results
Results for: 'pyruvic acid'
By: HWC, Views: 5790
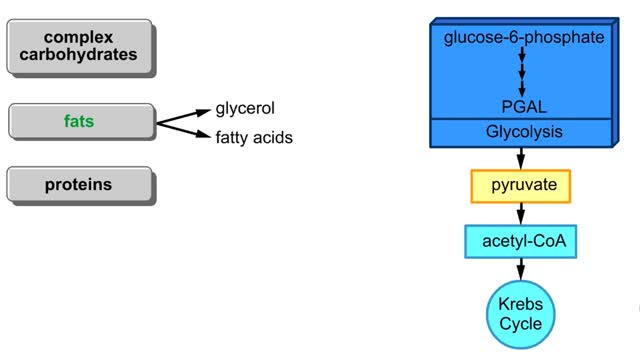
Points at which organic compounds enter the reaction stages of aerobic respiration. Complex carbohydrates are broken down into simple sugars, such as glucose. They become the substrates for glycolysis. If your body doesn't need to burn glucose for energy, glucose-6-phosphate can be co...
By: HWC, Views: 11703
The endocrine system maintains many body conditions within normal limits with feedback loops. Each endocrine feedback loop maintains homeostasis using the following components: • Stimulus - a change in a body condition. • Production cell - an endocrine cell that produces a hormone after b...
Plant Defense Mechanisms from Pathogens
By: HWC, Views: 11001
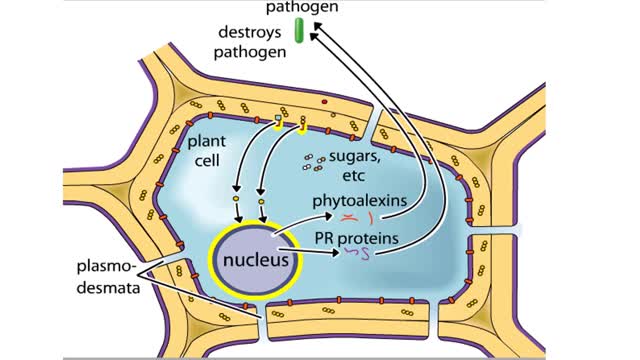
Plants and pathogens have coevolved such that pathogens can recognize plants by the sugars, or other molecules, they produce. Plants, in turn, can recognize pathogens by the molecules they produce. The ability to recognize pathogens allows plants to activate defense systems that can prevent wides...
Gastroesophageal Reflux Disease (GERD)
By: Administrator, Views: 14554
Gastroesophageal reflux disease, or GERD, is a digestive disorder that affects the lower esophageal sphincter (LES), the ring of muscle between the esophagus and stomach. Many people, including pregnant women, suffer from heartburn or acid indigestion caused by GERD.
Peptide Bond Formation Animation
By: HWC, Views: 5405
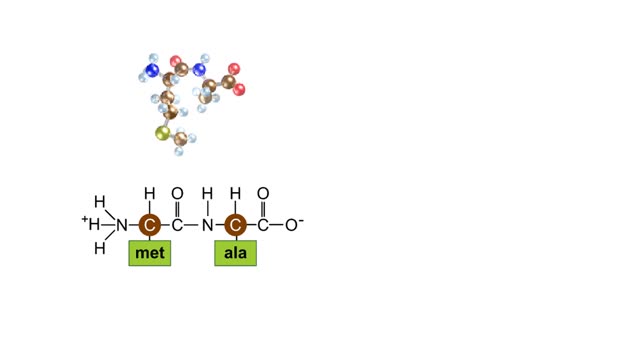
During protein synthesis, peptide bonds link amino acids together in the order specified by DNA instructions. In this case, the first two amino acids in the protein are methionine and alanine. Here are ball-and-stick models of these amino acids. Peptide bond formation is a type of condensatio...
By: HWC, Views: 11094
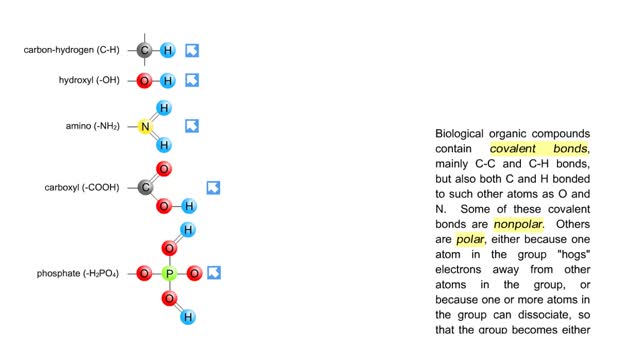
Biological organic compounds contain covalent bonds, mainly C-C and C-H bonds, but also both C and H bonded to such other atoms as O and N. Some of these covalent bonds are nonpolar. Others are polar, either because one atom in the group "hogs" electrons away from other atoms in the group, or...
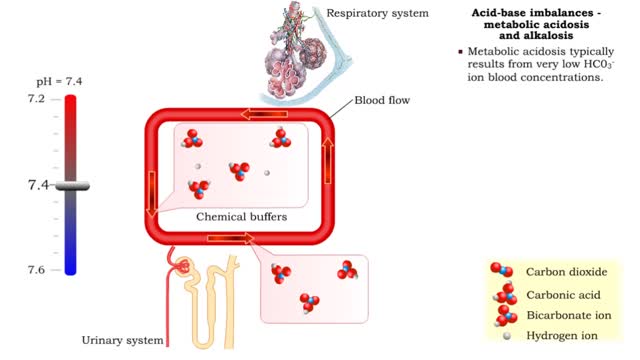
Acid-base imbalances - metabolic acidosis and alkalosis
By: HWC, Views: 11721
• Metabolic acidosis typically results from very low HCO3- ion blood concentrations. • Metabolic alkalosis typically results from very high HCO3- ion blood concentrations.
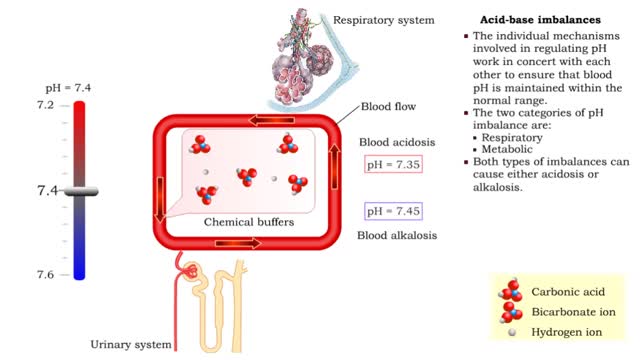
Acid-base imbalances - respiratory acidosis and alkalosis
By: HWC, Views: 11914
• The individual mechanisms involved in regulating pH work in concert with each other to ensure that blood pH is maintained within the normal range. • The two categories of pH imbalance are: • Respiratory • Metabolic • Both types of imbalances can cause either acidosis or alka...
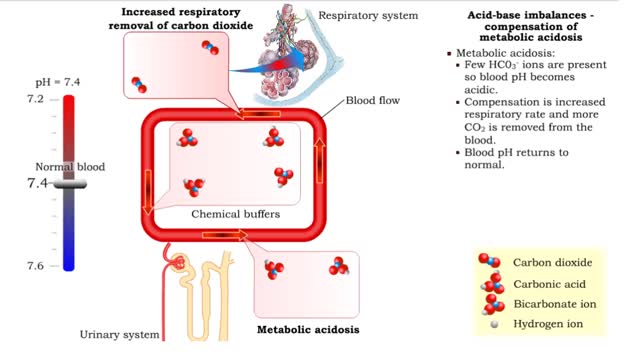
Acid-base imbalances - compensation of metabolic acidosis and alkalosis
By: HWC, Views: 11734
1. Metabolic acidosis: • Few HC03- ions are present so blood pH becomes acidic. • Compensation is increased respiratory rate and more CO2 is removed from the blood. • Blood pH returns to normal. 2. Metabolic alkalosis: • Many HC03- ions are present so blood pH becomes alkaline...
Advertisement