Search Results
Results for: 'Nucleic acid digestion'
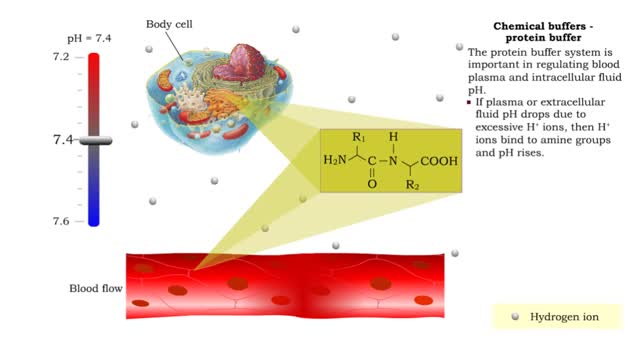
Chemical Buffers - protein buffer, phosphate buffer system and bicarbonate buffer system
By: HWC, Views: 11937
• There are a variety of chemicals in body fluids that prevent the fluids from undergoing large changes in. • These chemicals buffer or regulate fluctuations in H+ concentration. • Chemical buffers: • Bind to H+ ions when there are too many in a solution so pH remains normal. •...
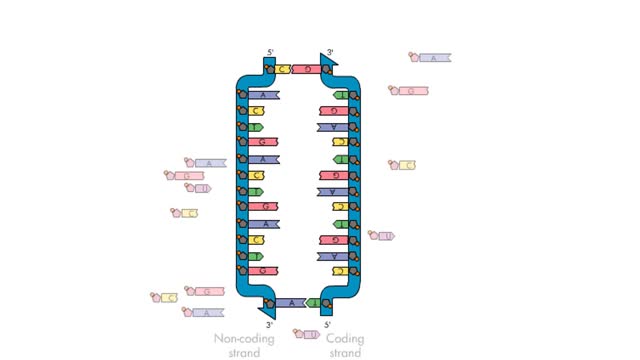
Transcription—A molecular view
By: HWC, Views: 7418
Transcription, as related to genomics, is the process of making an RNA copy of a gene's DNA sequence. This copy, called messenger RNA (mRNA), carries the gene's protein information encoded in DNA. During transcription, a DNA molecule is copied into RNA molecules that are then used to translate...
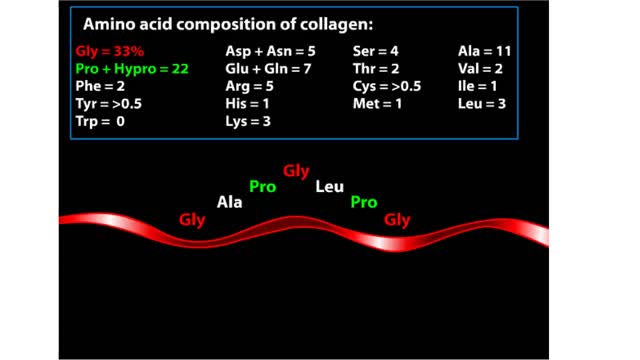
Major Elements in Biological Molecules: Proteins
By: HWC, Views: 11225
Proteins are chains of amino acids linked by peptide bonds. The 20 different amino acids used to make all proteins differ only in their side chains, and the properties of these side chains account for the great diversity of protein structure and function. Collagen is an example of how a prote...
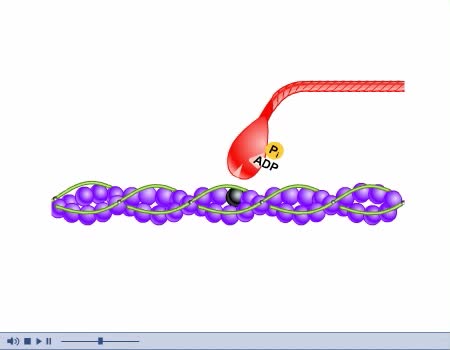
Contraction and Relaxation Animation
By: Administrator, Views: 14674
Muscles are responsible for movement. The types of movement are: - Locomotion, when chemical energy is changed into mechanical energy. - Propulsion of substances through tubes, as in circulation and digestion. - Changes in the sizes of openings, as in the contraction and relaxation of the iris...
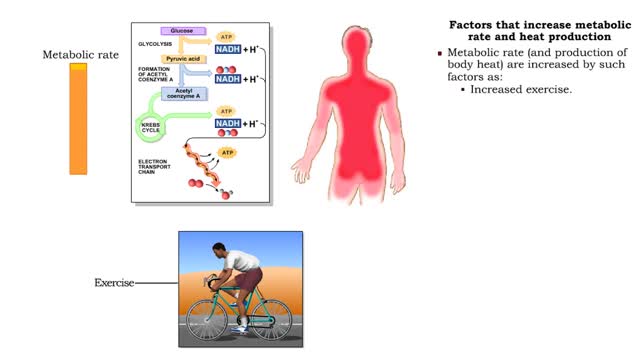
Factors that increase metabolic rate and heat production
By: HWC, Views: 11881
• All vital biochemical reactions are temperature dependent. • The overall rate at which metabolic reactions use energy is known as the metabolic rate. • Metabolic rate greatly determines body temperatures. • Temperature is maintained by balancing the loss of heat to the environment...
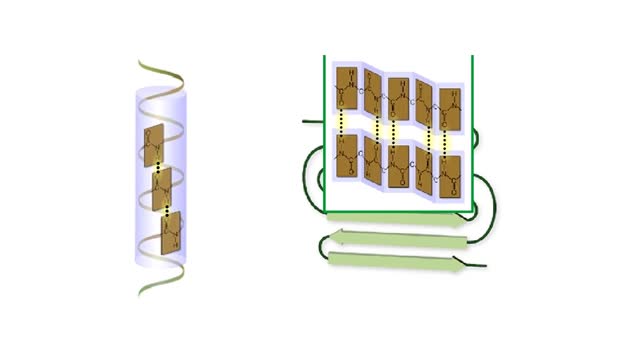
Secondary and tertiary levels of protein structure Animation
By: HWC, Views: 5655
Amino acid sequence dictates a protein's final shape. The presence of certain amino acids favors a pattern of hydrogen bonding that causes part of the polypeptide chain to coil and twist into an alpha helix. The presence of other amino acids enables hydrogen bonding between strand like re...
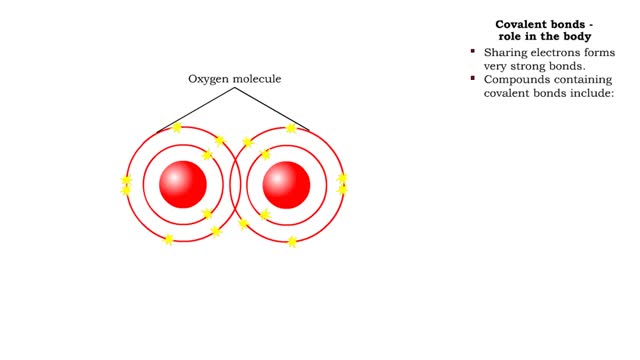
Covalent bonds - role in the body
By: HWC, Views: 11694
A covalent bond is formed when atoms share one or more pairs of electrons. This is opposed to an ionic bond, where electrons are actually transferred from one atom to another. Formation • Atoms fill up the outer orbital by sharing electrons. • Two oxygen atoms sharing electrons form on...
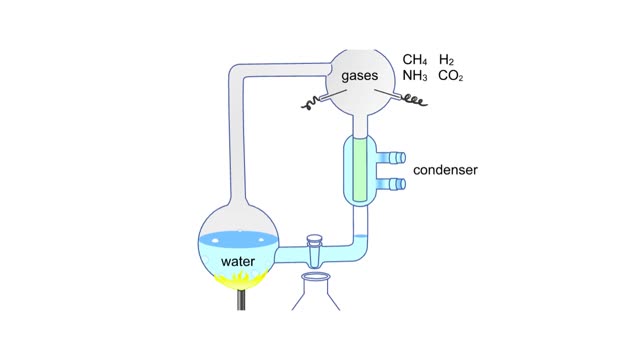
Miller's reaction chamber experiment Animation
By: HWC, Views: 5471
A simple diagram of Stanley Miller and Harold Urey's experimental apparatus. The lower portion of the apparatus was filled with water. The upper portion was filled with a mixture of gases that simulated the earth's early atmosphere. Examples are methane, ammonia, hydrogen and carbon dioxide. ...
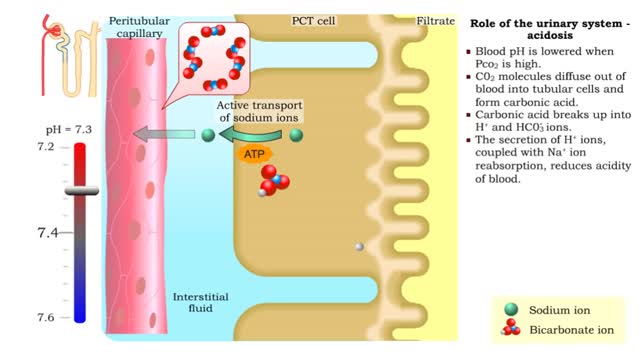
Role of the urinary system - acidosis and alkalosis
By: HWC, Views: 11943
• Tubular cells of the proximal convoluted tubule and collecting tubules can alter filtrate pH and therefore blood pH. • These cells can affect blood pH with two coupled mechanisms: • Reabsorption of bicarbonate ions. • Secretion of hydrogen ions. • The reabsorption of bicarbonate...
Advertisement