Non-polar compounds - insolubility
By: HWC
Date Uploaded: 10/16/2019
Tags: homeworkclinic.com Homework Clinic HWC insolubility Non-polar compounds non-polar molecule fatty acids Lipids partial charge water barrier Phospholipids hydrophobic hydrophilic دهن محب للماء المركبات غير القطبية
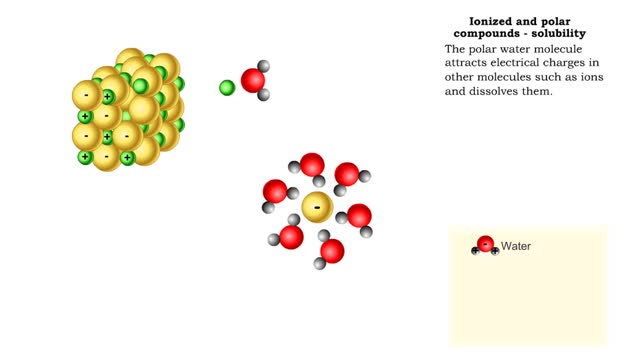
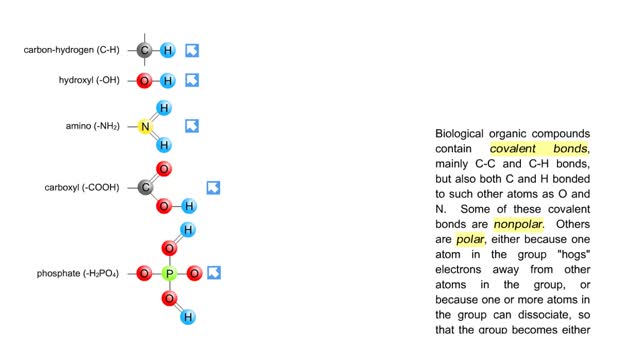
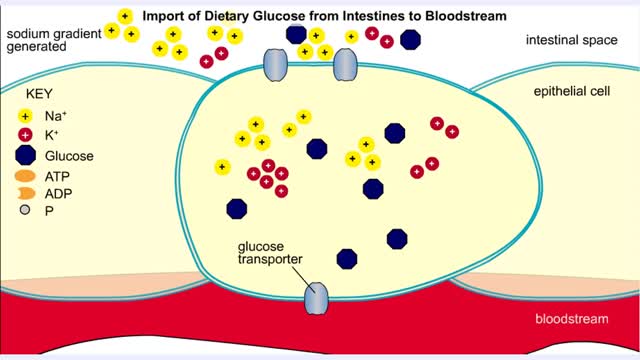
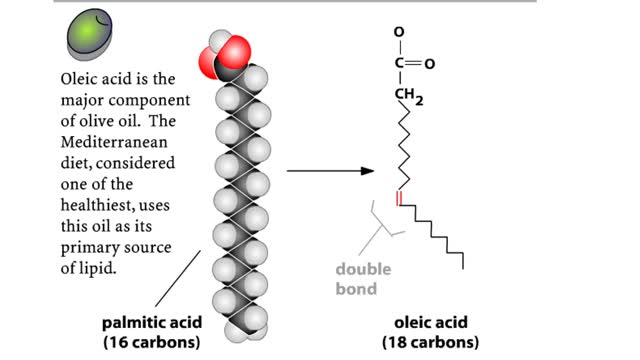
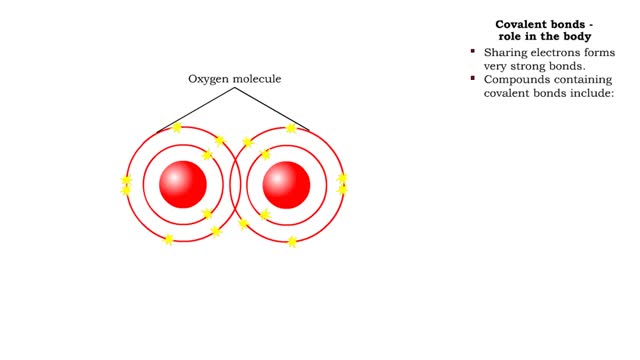
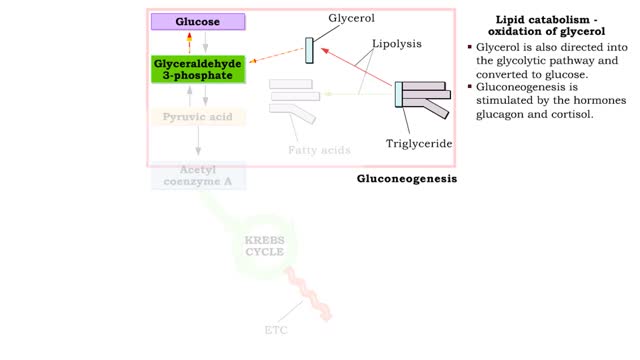
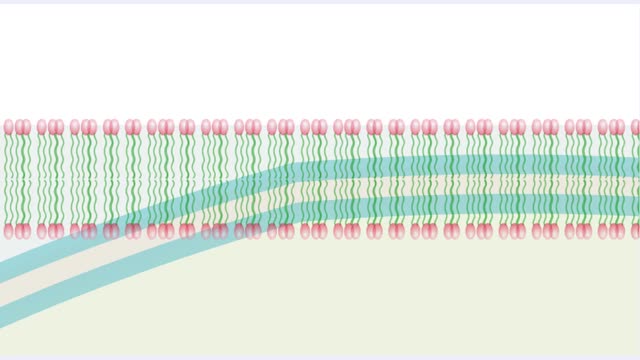
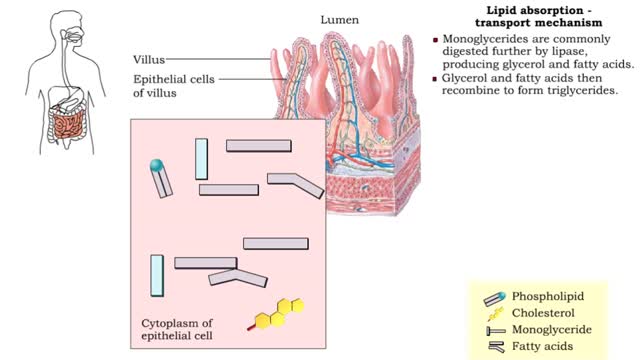
• A non-polar molecule has uniform distribution of electrons. • Non-polar compounds like fatty acids in lipids have a high proportion of carbon and hydrogen. • Lipids possess no charge or partial charge. • Lipids are not attracted to water molecules. • Lipids are not soluble in water. Phospholipids - water barrier ■ Lipids are important components of the cell membrane. ■ Lipids separate two watery solutions without dissolving. ■ The hydrophobic, non-polar lipid tail moves away from the water inside and outside of the cell. ■ The hydrophilic, polar head mixes with water. ■ A double layer of phospholipids in cell membranes forms a waterproof barrier.
Add To
You must login to add videos to your playlists.
Advertisement




Comments
0 Comments total
Sign In to post comments.
No comments have been posted for this video yet.