The life cycle of protein
By: HWC
Date Uploaded: 06/18/2020
Tags: homeworkclinic.com Homework Clinic HWC The life cycle of protein ribosome polypeptide chain chaperone protein ATP cytoskeleton N-terminus PEST conjugating enzyme 26S proteasome cytoplasm ubiquitin chain
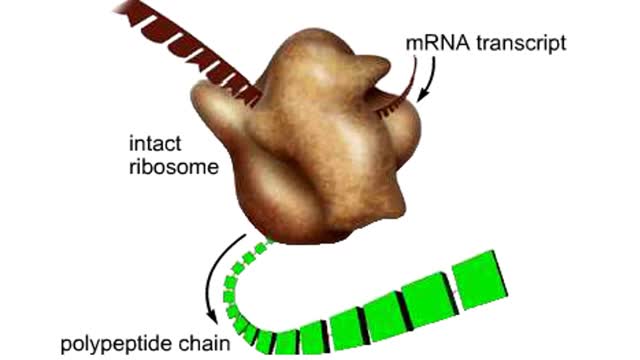
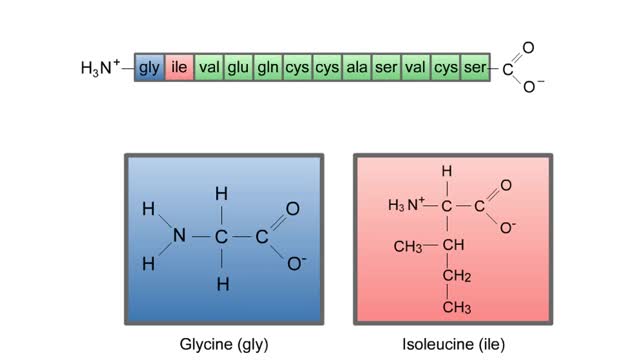
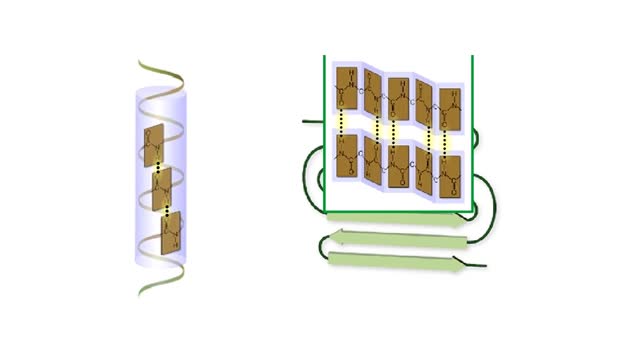
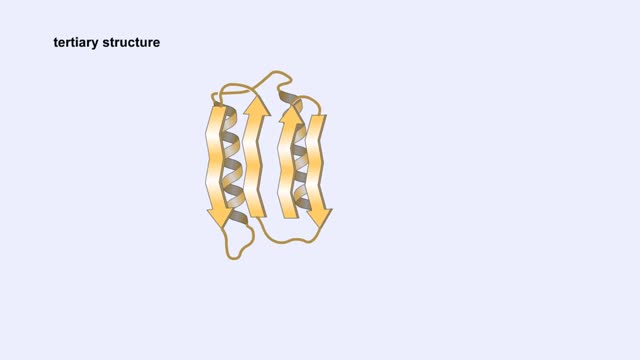
The life cycle of a typical protein begins with its synthesis on a ribosome. As the polypeptide chain grows, molecules of a chaperone protein bind along its length. This prevents misfolding of the nascent polypeptide. ATP binding causes chaperone release. For most proteins, the polypeptide then folds into a native conformation. However, for some proteins, like actin, an additional folding step occurs. The unfolded polypeptide is bound in a large chaperonin protein. Within the chaperonin, the polypeptide folds and is expelled from the complex. This process requires ATP. The newly liberated actin is then able to polymerize into a filament that becomes part of the actin cytoskeleton. The lifespan of a protein is determined by the amino acid at its N-terminus or by the presence of a destruction sequence, such as PEST. Protein degradation begins when ubiquitin is covalently attached to the target protein by a conjugating enzyme. Multiple rounds of this reaction modifies the protein with a ubiquitin chain.The chain targets the protein for the 26S proteasome, the major protein degradation machine in the cytoplasm. The proteasome cleaves the tagged protein into ubiquitin and short peptides.
Add To
You must login to add videos to your playlists.
Advertisement



Comments
0 Comments total
Sign In to post comments.
No comments have been posted for this video yet.