Miller's reaction chamber experiment Animation
By: HWC
Date Uploaded: 11/30/2023
Tags: homeworkclinic.com Miller's reaction chamber experiment Animation Harold Urey's experimental Stanley Miller methane ammonia hydrogen carbon dioxide condenser Electrodes chamber boiling point amino acids glycine alanine aspartic acid glutamic organic compounds
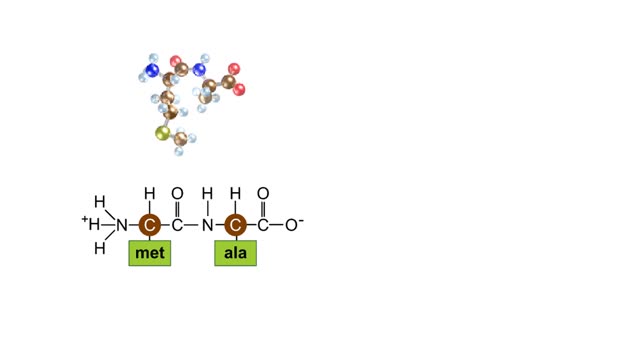
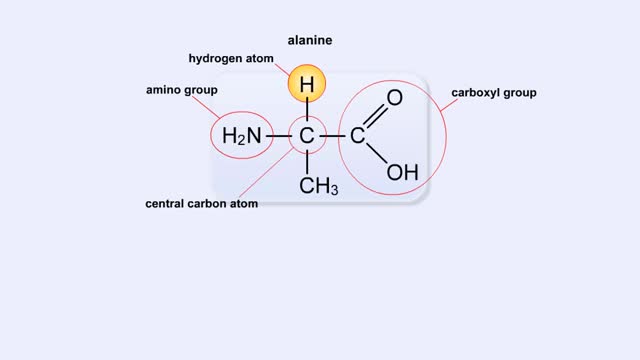
A simple diagram of Stanley Miller and Harold Urey's experimental apparatus. The lower portion of the apparatus was filled with water. The upper portion was filled with a mixture of gases that simulated the earth's early atmosphere. Examples are methane, ammonia, hydrogen and carbon dioxide. Electrodes extended into the reaction chamber and could be used to produce electrical sparks. A condenser, through which cold water flowed, cooled the portion of the glass tube below the spark chamber. To begin the experiment, the water in the lower chamber was heated to the boiling point. Water vapor began to circulate through the upper chamber, mixing with the gases in the presence of the electrical sparks. As the vapor passed through the cooled area, droplets formed and accumulated in the lower portion of the apparatus. The mixture kept circulating through the chamber while being bombarded with electricity. To test the effects of this treatment a sample of fluid was removed from the chamber. Chemical analysis of the fluid revealed the presence of the amino acids glycine, alanine, aspartic acid, glutamic acid and other organic compounds. These molecules formed in less than a week. The results suggested that the complex organic molecules characteristic of life could have formed under conditions that existed on the early earth.
Add To
You must login to add videos to your playlists.
Advertisement



Comments
0 Comments total
Sign In to post comments.
No comments have been posted for this video yet.