Search Results
Results for: 'Partial positive charge'
Hydrogen bonds - role in the body
By: HWC, Views: 10813
A hydrogen bond is the electromagnetic attraction between polar molecules in which hydrogen is bound to a larger atom, such as oxygen or nitrogen. This is not a sharing of electrons, as in a covalent bond. Instead, this is an attraction between the positive and negative poles of charged atoms. ...
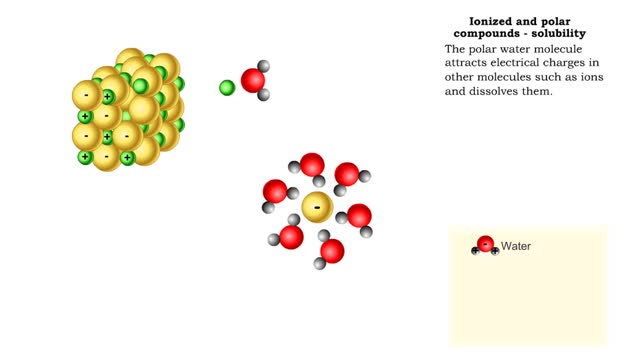
Properties of water -structure of water and polarity (Ionized and polar compounds)
By: HWC, Views: 10670
■ Water transports most of the molecules in the body. ■ The structure of a water molecule allows it to dissolve other molecules. ■ Shared electrons spend more time near the oxygen atom. ■ Oxygen end has a partial negative charge. ■ Hydrogen ends have a partial positive charge....
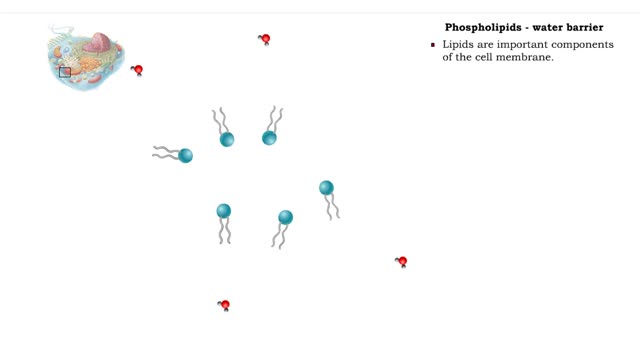
Non-polar compounds - insolubility
By: HWC, Views: 10586
• A non-polar molecule has uniform distribution of electrons. • Non-polar compounds like fatty acids in lipids have a high proportion of carbon and hydrogen. • Lipids possess no charge or partial charge. • Lipids are not attracted to water molecules. • Lipids are not soluble in...
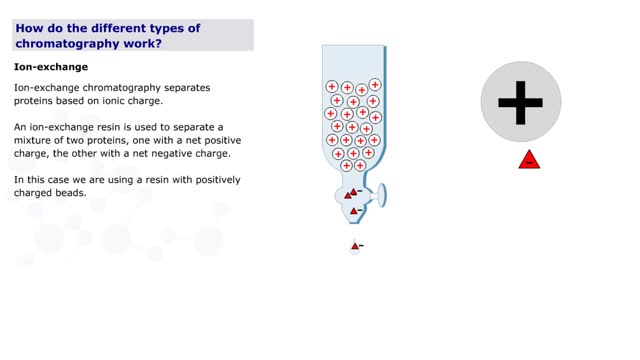
How do the different types of chromatography work? (No Audio)
By: HWC, Views: 9882
Chromatography is a term for a variety of techniques in which a mixture of dissolved components is fractionated as it moves through some type of porous matrix. A glass column is filled with beads of an inert matrix. The mixture of proteins to be purified is dissolved in a solution and passed ...
Bond in biological molecules (Ionic, Covalent and Hydrogen bonds)/ How atoms bond?
By: HWC, Views: 7817
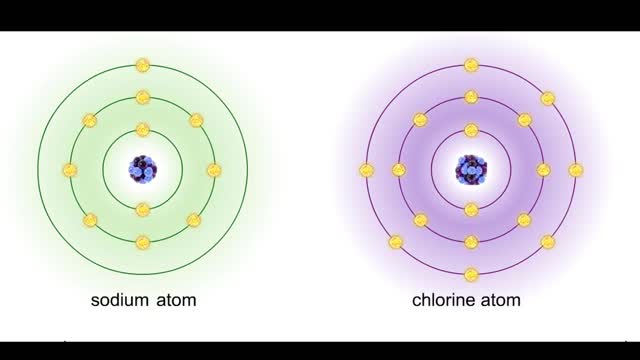
Sodium atoms and chloride atoms have unfilled orbitals in their outer shells. The lone electron in the outermost shell of a sodium atom can be pulled or knocked out. This ionizes the atom. It is now a positively charged sodium ion. A chlorine atom has an electron vacancy in its outer shell and...
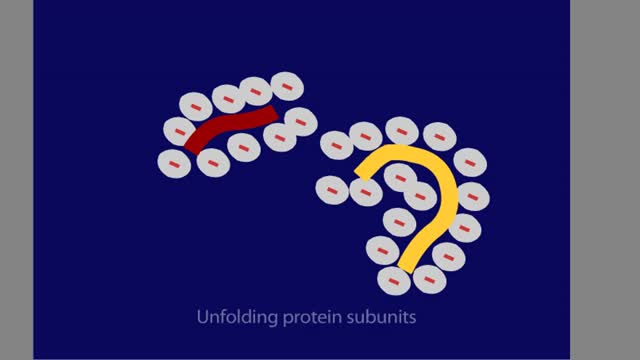
Power Supply Polyacrylamide Gel Protein Sample
By: HWC, Views: 9806
SDS-polyacrylamide gel electrophoresis is a powerful tool, which resolves proteins according to their molecular weights. Because proteins differ in size, shape, and charge, a protein sample is first denatured with the anionic detergent SDS. When the sample is heated, the SDS molecules bind to ...
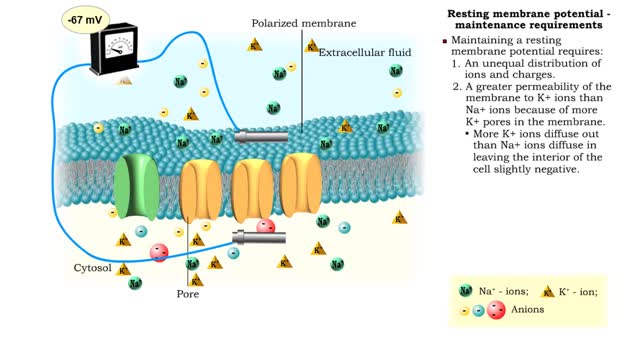
Resting membrane potential - electrical polarity and maintenance requirements
By: HWC, Views: 10199
• A resting membrane potential exists when there is a buildup of: 1. positive ions outside the membrane. 2. negative ions inside the membrane. • Membranes with opposing charges are said to be polarized. • The difference in charge applies only to the small distance across the membran...
By: HWC, Views: 9990
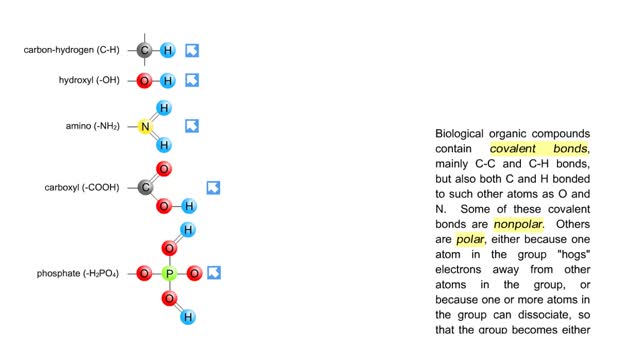
Biological organic compounds contain covalent bonds, mainly C-C and C-H bonds, but also both C and H bonded to such other atoms as O and N. Some of these covalent bonds are nonpolar. Others are polar, either because one atom in the group "hogs" electrons away from other atoms in the group, or...
By: HWC, Views: 9277
The slight positive charge of a hydrogen atom in a water molecule can attract an atom with a slight negative charge, such as the nitrogen in a molecule of ammonia. This forms a hydrogen bond between the two atoms. Hydrogen bonds join the two strands of a DNA molecule. Although hydrogen bo...
Advertisement