Search Results
Results for: 'peptide bonds'
Peptide Bond Formation Animation
By: HWC, Views: 4950
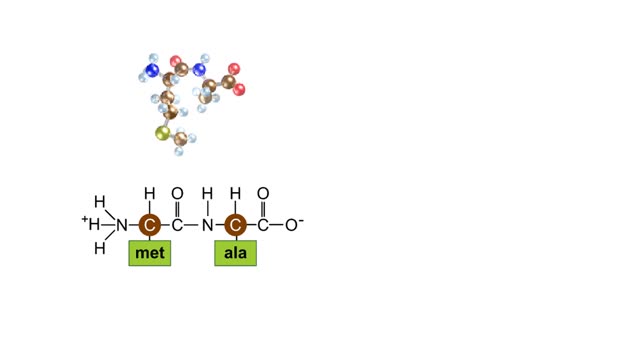
During protein synthesis, peptide bonds link amino acids together in the order specified by DNA instructions. In this case, the first two amino acids in the protein are methionine and alanine. Here are ball-and-stick models of these amino acids. Peptide bond formation is a type of condensatio...
Structure of Amino Acid, Peptide Bonds & Polypeptides
By: HWC, Views: 10762
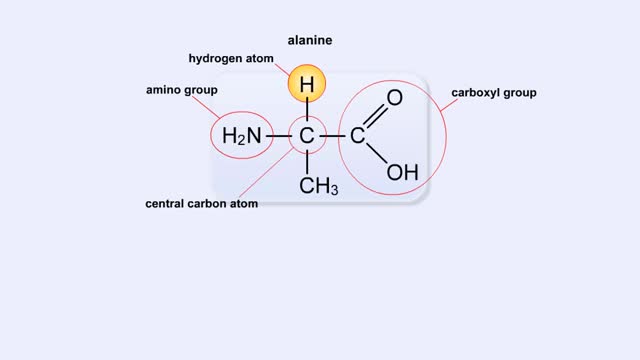
Here are the molecular formulas of three different amino acids. All amino acids share this backbone. The main difference between every amino acid is the side groups seen here, and these side groups give each of the amino acids their different characteristics. But before we get into that, let's ...
Major Elements in Biological Molecules: Proteins
By: HWC, Views: 10675
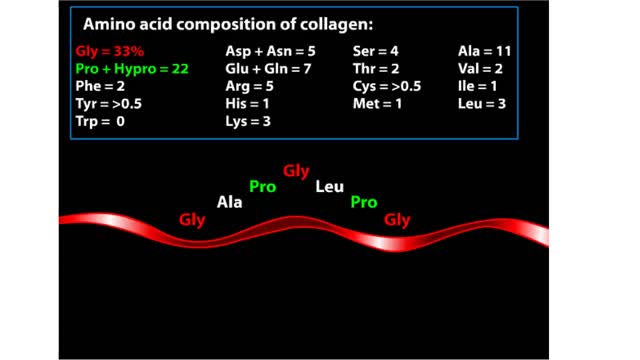
Proteins are chains of amino acids linked by peptide bonds. The 20 different amino acids used to make all proteins differ only in their side chains, and the properties of these side chains account for the great diversity of protein structure and function. Collagen is an example of how a prote...
Protein Structure - Primary, Secondary, Tertiary and Quaternary
By: HWC, Views: 11041
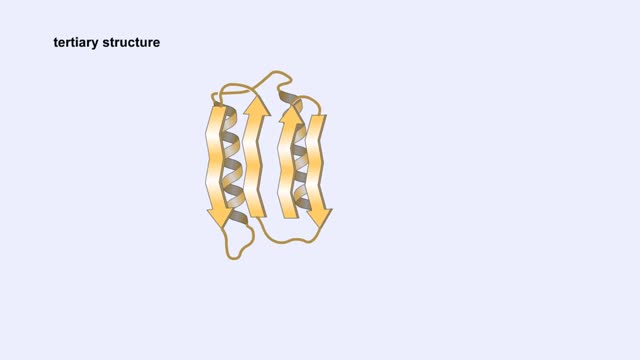
A protein's first order structure, or primary structure, begins with the amino acid sequence of the polypeptide chain. The 20 different amino acids can be arranged in an infinite number of sequences. For example, the hormone insulin, which regulates the uptake of glucose from the blood into ce...
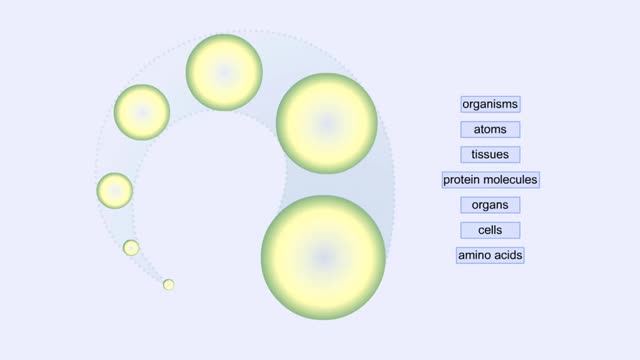
Proteins Defined, Hierarchy & Composition of Cells
By: HWC, Views: 10654
Proteins are long chains of amino acids linked together by peptide bonds. Together with the other three biological macromolecules—carbohydrates, lipids, and nucleic acids—proteins are the building blocks of cells. Proteins are the most complex and abundant biological macromolecules in cel...
By: HWC, Views: 9953
The slight positive charge of a hydrogen atom in a water molecule can attract an atom with a slight negative charge, such as the nitrogen in a molecule of ammonia. This forms a hydrogen bond between the two atoms. Hydrogen bonds join the two strands of a DNA molecule. Although hydrogen bo...
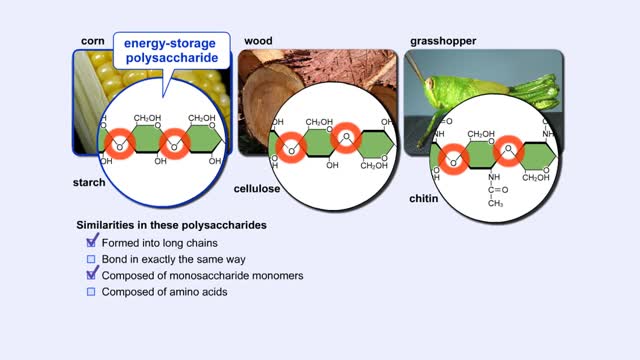
By: HWC, Views: 10761
More complex sugars are called polysaccharides (from "poly" meaning "many" and "saccharum" meaning "sugar"). Many things in nature are made of polysaccharides. Here we show one of the polysaccharides in corn, another in wood, and another in the exoskeletons of insects like grasshoppers. How are a...
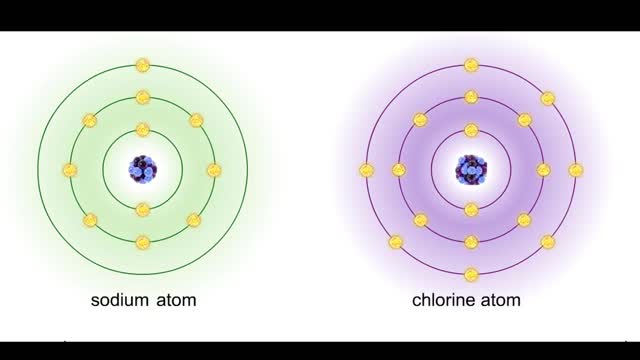
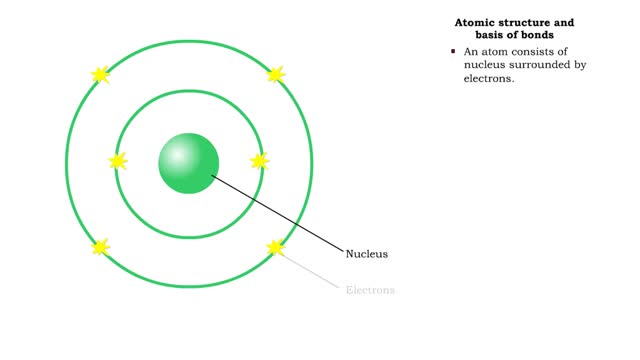
Bond types - Atomic structure and basis of bonds
By: HWC, Views: 11567
• Chemical bonds are fundamental to the structure and function of many types of molecules, such as proteins, carbohydrates, lipids, nucleic acids, gases, salts and water. ■ These molecules are composed of atoms that are held together by three different types of bonds. • The three types ...
Hydrogen bonds - role in the body
By: HWC, Views: 11467
A hydrogen bond is the electromagnetic attraction between polar molecules in which hydrogen is bound to a larger atom, such as oxygen or nitrogen. This is not a sharing of electrons, as in a covalent bond. Instead, this is an attraction between the positive and negative poles of charged atoms. ...
Advertisement