Search Results
Results for: 'ionic bonds'
By: HWC, Views: 11262
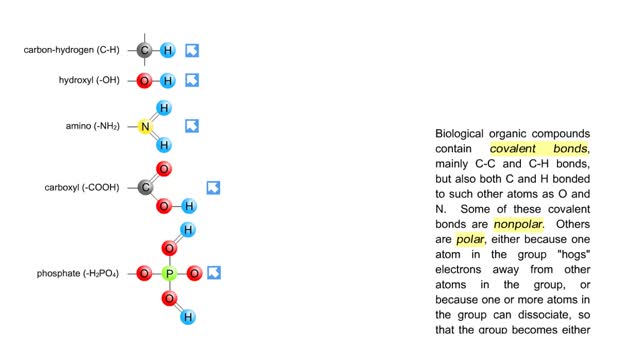
Biological organic compounds contain covalent bonds, mainly C-C and C-H bonds, but also both C and H bonded to such other atoms as O and N. Some of these covalent bonds are nonpolar. Others are polar, either because one atom in the group "hogs" electrons away from other atoms in the group, or...
Anatomy and Chemical Makeup of a Single Hair (Animation)
By: HWC, Views: 10122
The hair's outer cuticle surrounds hair cells filled with tough keratin macrofibrils. Each macrofibril consists of smaller microfibrils. A microfibril is made up of three keratin polypeptide chains. The chains are linked together by disulfide bonds. A hair consists of keratin chains held...
Activation Energy - Valence Electrons
By: HWC, Views: 11200
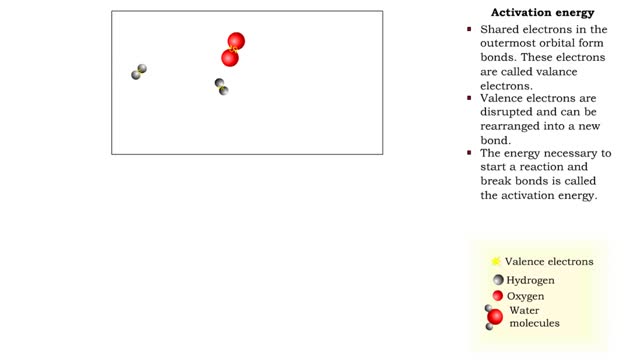
■ Shared electrons in the outermost orbital form bonds. These electrons are called valence electrons. ■ Valence electrons are disrupted and can be rearranged into a new bond. ■ The energy necessary to start a reaction and break bonds is called the activation energy. ■ Reactants have...
Major Elements in Biological Molecules: Lipids
By: HWC, Views: 11120
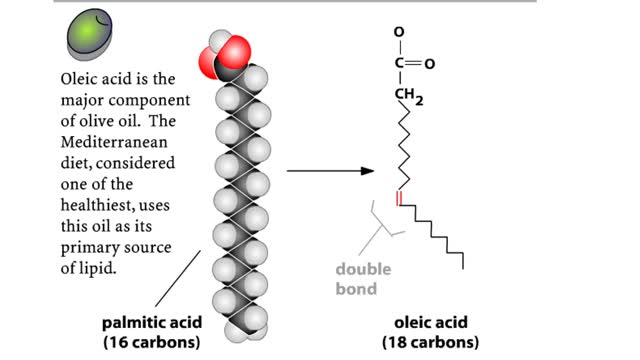
A triglyceride (also called triacylglycerol) is composed of three fatty acid molecules and one glycerol molecule. The fatty acids attach to the glycerol molecule by a covalent ester bond. The long hydrocarbon chain of each fatty acid makes the triglyceride molecule nonpolar and hydrophobic. Pa...
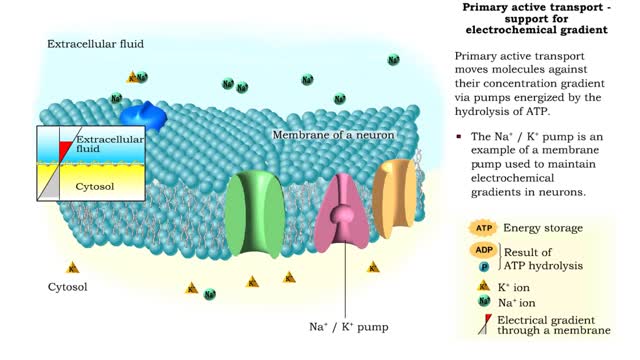
Primary Active Transport - electrochemical gradient and ion transport / water movement
By: HWC, Views: 11952
Energy derived from ATP changes the shape of a transporter protein which pumps a substance across a plasma membrane against its concentration gradient An electrochemical gradient is a gradient of electrochemical potential, usually for an ion that can move across a membrane. The gradient consis...
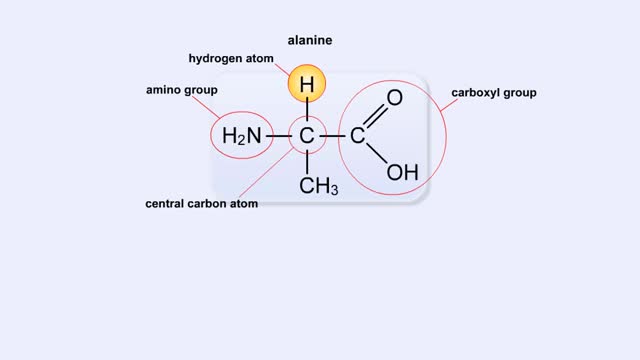
Structure of Amino Acid, Peptide Bonds & Polypeptides
By: HWC, Views: 11320
Here are the molecular formulas of three different amino acids. All amino acids share this backbone. The main difference between every amino acid is the side groups seen here, and these side groups give each of the amino acids their different characteristics. But before we get into that, let's ...
Neurotransmission at chemical synapses & Excitory and inhibitory potentials
By: HWC, Views: 11708
• A series of events occur at chemical synapses in order to communicate with the adjacent cell. • The action potential arrives at the presynaptic membrane. • The depolarization phase of the action potential opens voltage gated Ca+ channels. • increased inflow of Ca+' into the cyto...
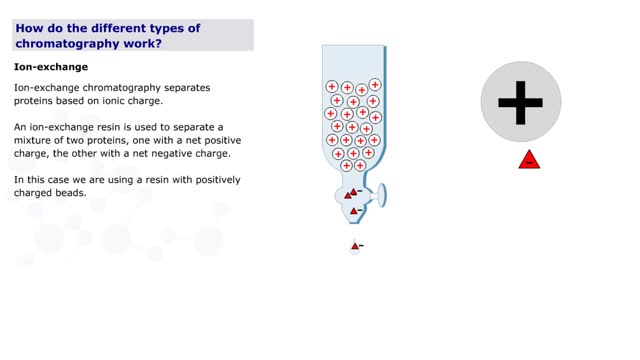
How do the different types of chromatography work? (No Audio)
By: HWC, Views: 11180
Chromatography is a term for a variety of techniques in which a mixture of dissolved components is fractionated as it moves through some type of porous matrix. A glass column is filled with beads of an inert matrix. The mixture of proteins to be purified is dissolved in a solution and passed ...
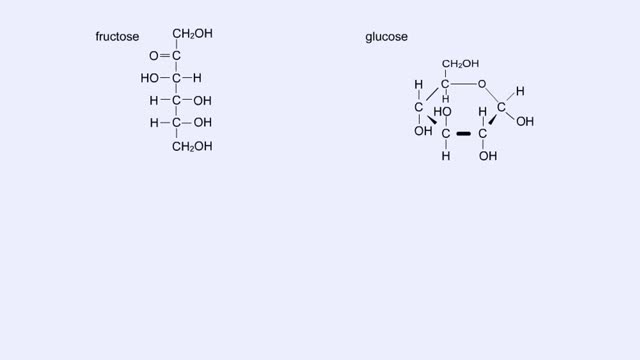
By: HWC, Views: 11491
Here are the molecular structures of three simple sugars: glucose, ribose, and fructose. Look at these simple sugars and identify what characteristics they all share. As you can see, all of the carbohydrates have carbon, hydrogen, and oxygen in a ratio of 1:2:1 and there is always a double bo...
Advertisement