Search Results
Results for: 'ribulose bisphosphate molecule'
By: HWC, Views: 10521
The slight positive charge of a hydrogen atom in a water molecule can attract an atom with a slight negative charge, such as the nitrogen in a molecule of ammonia. This forms a hydrogen bond between the two atoms. Hydrogen bonds join the two strands of a DNA molecule. Although hydrogen bo...
Bond in biological molecules (Ionic, Covalent and Hydrogen bonds)/ How atoms bond?
By: HWC, Views: 8997
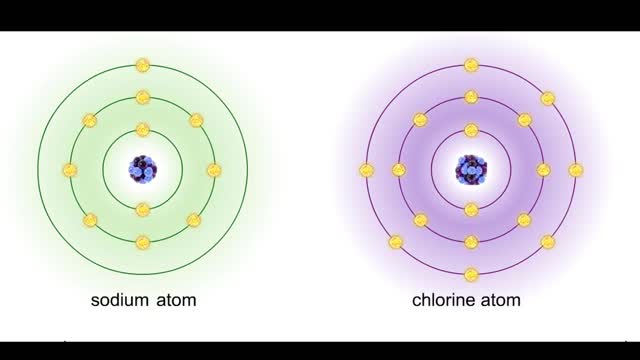
Sodium atoms and chloride atoms have unfilled orbitals in their outer shells. The lone electron in the outermost shell of a sodium atom can be pulled or knocked out. This ionizes the atom. It is now a positively charged sodium ion. A chlorine atom has an electron vacancy in its outer shell and...
Hydrogen bonds - role in the body
By: HWC, Views: 11993
A hydrogen bond is the electromagnetic attraction between polar molecules in which hydrogen is bound to a larger atom, such as oxygen or nitrogen. This is not a sharing of electrons, as in a covalent bond. Instead, this is an attraction between the positive and negative poles of charged atoms. ...
What are the Parts of a Plant Cell?
By: HWC, Views: 10763
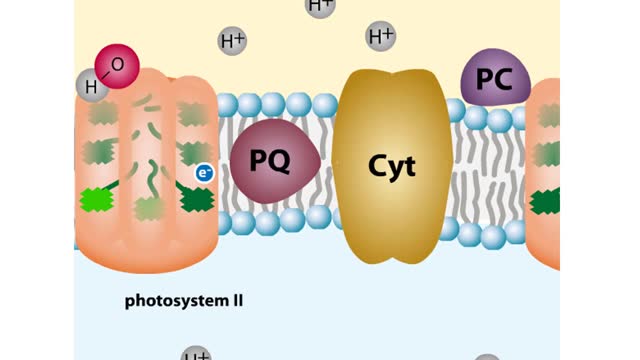
Every chloroplast in a plant cell is packed with stacks of flattened sacs called thylakoids. The thylakoid membranes contain chlorophyll, as well as most of the other components required for the light reactions of photosynthesis. The chlorophyll-containing structures within the membranes are c...
Major Elements in Biological Molecules: Lipids
By: HWC, Views: 11111
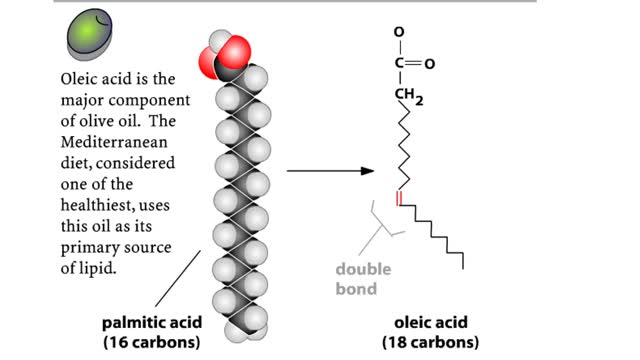
A triglyceride (also called triacylglycerol) is composed of three fatty acid molecules and one glycerol molecule. The fatty acids attach to the glycerol molecule by a covalent ester bond. The long hydrocarbon chain of each fatty acid makes the triglyceride molecule nonpolar and hydrophobic. Pa...
Properties of water -structure of water and polarity (Ionized and polar compounds)
By: HWC, Views: 11863
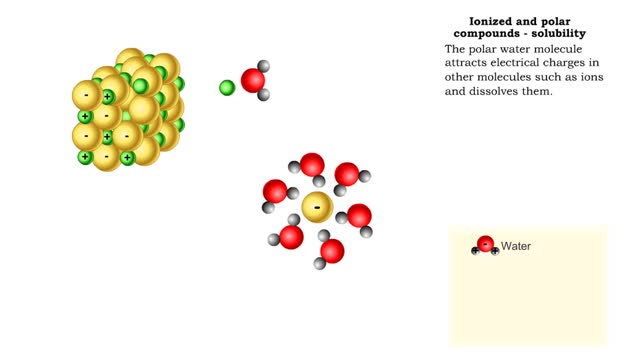
■ Water transports most of the molecules in the body. ■ The structure of a water molecule allows it to dissolve other molecules. ■ Shared electrons spend more time near the oxygen atom. ■ Oxygen end has a partial negative charge. ■ Hydrogen ends have a partial positive charge....
Sister chromatids of a metaphase chromosome animation
By: HWC, Views: 9906
At metaphase, the chromosomes are duplicated and are at their most condensed. In each chromosome. two identical sister chromatids are held together at a constricted region called the centromere. When a chromosome is condensed, interactions among chromosomal proteins keep loops of DNA tightly ...
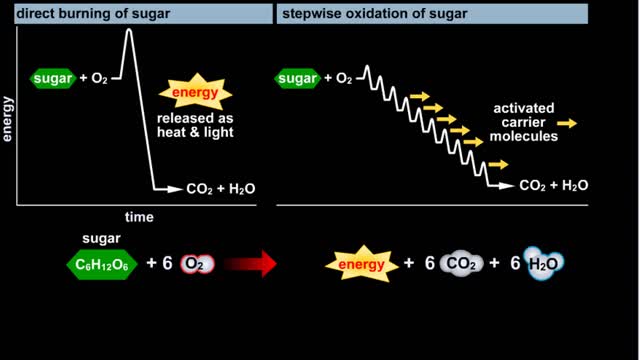
Glycolysis - Introduction to ATP and the burning of sugar
By: HWC, Views: 11967
Do you use sugar with your coffee or tea? Or do you occasionally drink a sport or soft drink? As millions of people do each day, they obtain energy from the sugar added or contained in these drinks. How can we understand this concept of energy within a sugar molecule? Let's take a tablespoon ...
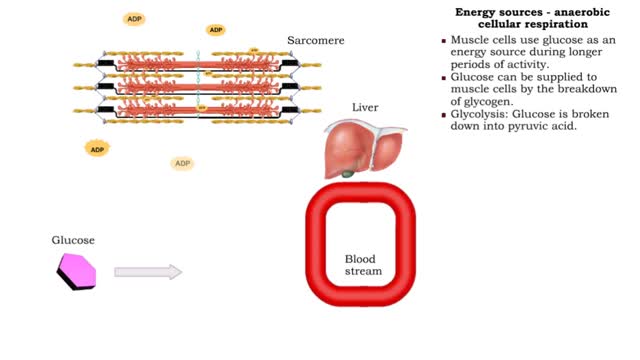
By: HWC, Views: 11956
• The amount of ATP stored in a skeletal muscle cell can only provide muscular activity for two to three seconds. • Muscle cells must be able to generate additional molecules of ATP to continue contracting. • Muscle cells can generate ATP from several processes: • Phosphogen syste...
Advertisement