Search Results
Results for: 'El'
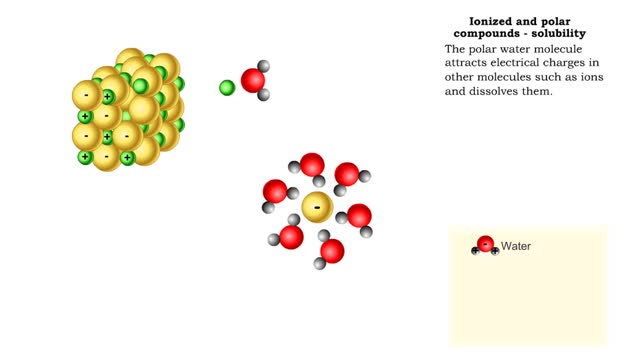
Properties of water -structure of water and polarity (Ionized and polar compounds)
By: HWC, Views: 11075
■ Water transports most of the molecules in the body. ■ The structure of a water molecule allows it to dissolve other molecules. ■ Shared electrons spend more time near the oxygen atom. ■ Oxygen end has a partial negative charge. ■ Hydrogen ends have a partial positive charge....
Non-polar compounds - insolubility
By: HWC, Views: 11057
• A non-polar molecule has uniform distribution of electrons. • Non-polar compounds like fatty acids in lipids have a high proportion of carbon and hydrogen. • Lipids possess no charge or partial charge. • Lipids are not attracted to water molecules. • Lipids are not soluble in...
By: HWC, Views: 10830
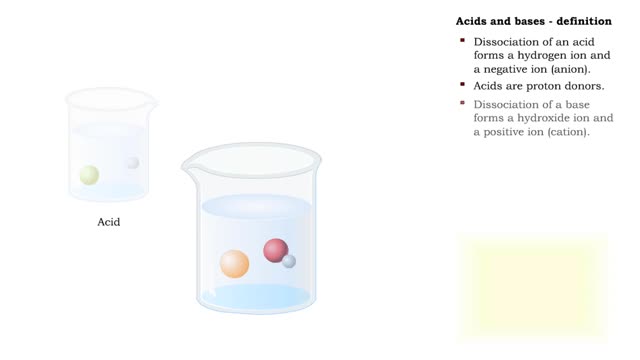
Acids and bases are found all around your house. For example, if you open your pantry or refrigerator, you might see a lot of acids. Fruit juice, soda pop, vinegar, and milk are all examples of acids. The word acid actually comes from a Latin term meaning ''sour.'' Many materials, like sugar for ...
The pH scale - Strong acids and Weak acids
By: HWC, Views: 11017
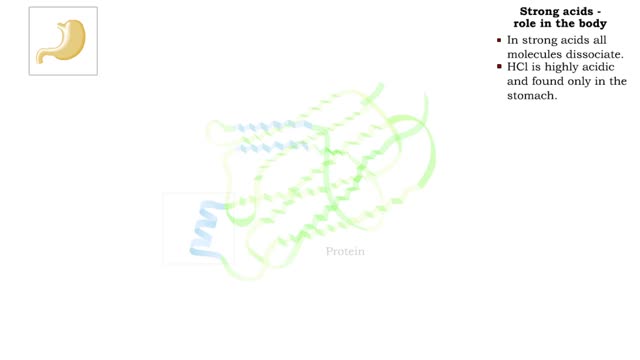
The pH scale • Expresses concentration of H+. • range: 0-14. • 7 is neutral. • Less 7 is acid. • greater 7 is basic (alkaline). Strong acids - role in the body ■ In strong acids all molecules dissociate. ■ HC1 is highly acidic and found only in the stomach. • H...
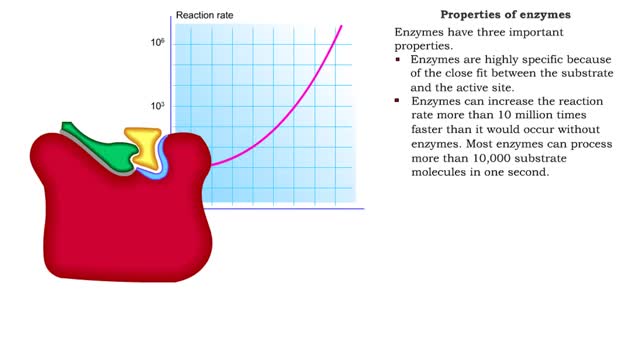
Enzyme structure - Properties of enzymes
By: HWC, Views: 10902
■ Enzymes are proteins that catalyze reactions. ■ Some enzymes have two parts: a protein or apoenzyme and a non-protein or cofactor. ■ Cofactor can be a metal ion or another organic molecule called a coenzyme. ■ Coenzymes often come from vitamins. ■ Cofactors affect the shape of...
By: HWC, Views: 10753
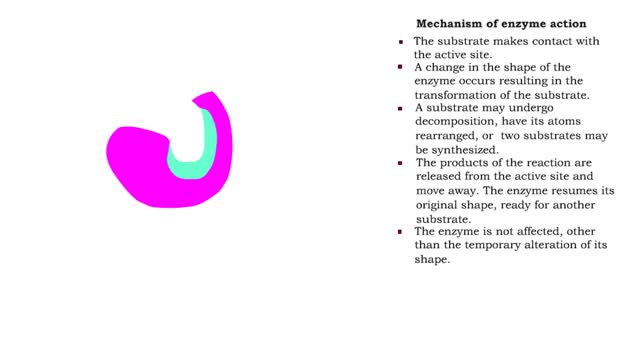
■ The substrate makes contact with the active site. ■ A change in the shape of the enzyme occurs resulting in the transformation of the substrate. ■ A substrate may undergo decomposition, have its atoms rearranged, or two substrates may be synthesized. ■ The products of the reaction...
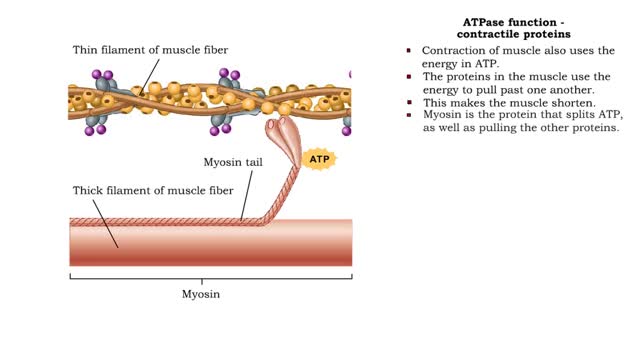
ATPase function - membrane transport, contractile proteins and synthesis
By: HWC, Views: 11374
• Energy from ATP is used to move ions across the cell membrane during active transport. • This membrane protein transports sodium out of the cell and potassium into the cell. As such, it is called a sodium-potassium pump. • Because this pump also acts as an enzyme to hydrolyze ATP it i...
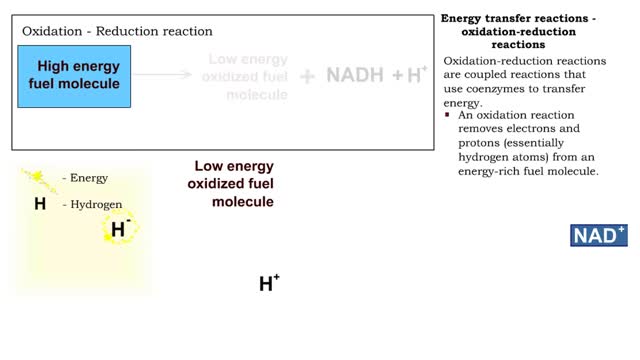
Types of energy transfer reactions: oxidation-reduction reactions and ATP generation reactions
By: HWC, Views: 11579
■ Metabolism balances anabolic and catabolic reactions. ■ Anabolism is energy transfer from ATP to simpler molecules in order to build them up into larger, more complex molecules. ■ Catabolism is breaking down larger, more complex molecules, usually to transfer energy from them in order...
By: HWC, Views: 11163
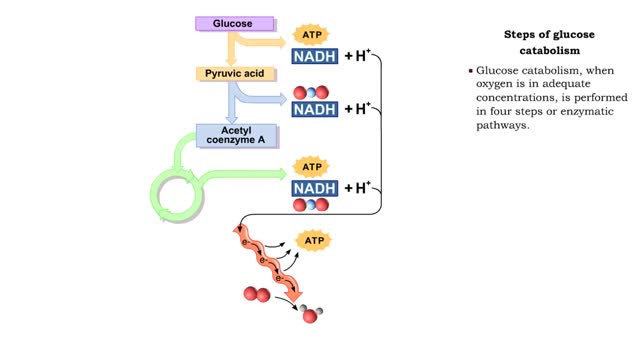
• During digestion, complex carbohydrates are hydrolyzed into monosaccharides, primarily glucose. • The catabolism of glucose is the primary source of energy for cellular production of ATP. • The anabolism of glucose is important in regulating blood glucose levels. • Glucose cat...
Advertisement