Search Results
Results for: 'disulfide bonds'
Buffers definition and the role of buffer in the body
By: HWC, Views: 11054
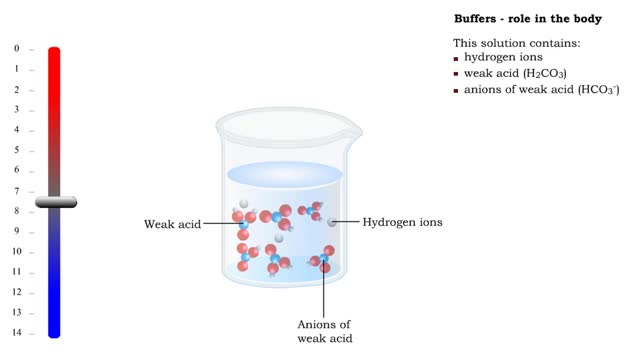
■ Too many H+ break hydrogen bonds and a protein comes apart. ■ Buffers react with excess H+ to protect proteins from breaking down. ■ Buffers consist of weak acid plus anions of that weak acid. This solution contains: • hydrogen ions • weak acid (H2CO3) • anions of we...
DNA Replication Factory and Protein
By: HWC, Views: 10634
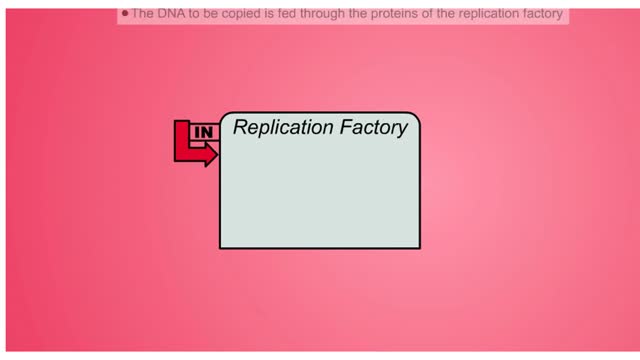
DNA (deoxyribose nucleic acid) carries all the genetic information needed to re-create itself and to pass on the characteristics of the organism. The “factory” model of DNA replication hypothesizes a specific nuclear structure in which the molecular machinery for replication forks are brou...
Protein catabolism (Krebs cycle) and Protein anabolism (protein synthesis)
By: HWC, Views: 11598
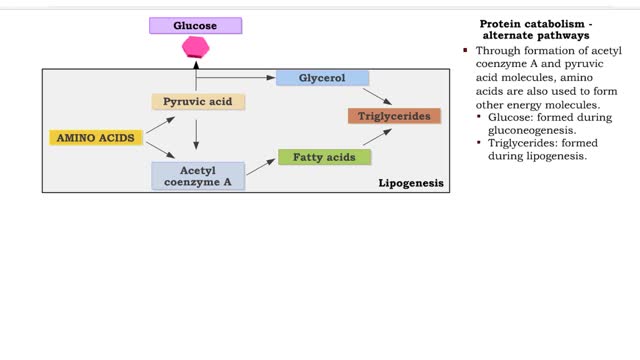
• Deaminated acids are brought into the Krebs cycle to be oxidized to CO2 and H2O. • Before entering the Krebs cycle, the deaminated acids are converted into intermediate products (pyruvic acid, acetyl coenzyme A, carbonic acids). • In the Krebs cycle, amino acids are oxidized to form r...
Glycolysis - Introduction to ATP and the burning of sugar
By: HWC, Views: 11274
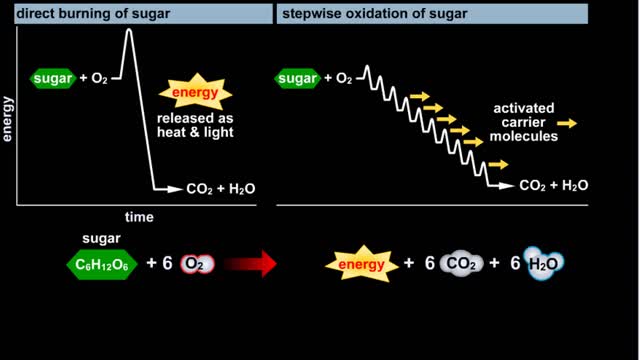
Do you use sugar with your coffee or tea? Or do you occasionally drink a sport or soft drink? As millions of people do each day, they obtain energy from the sugar added or contained in these drinks. How can we understand this concept of energy within a sugar molecule? Let's take a tablespoon ...
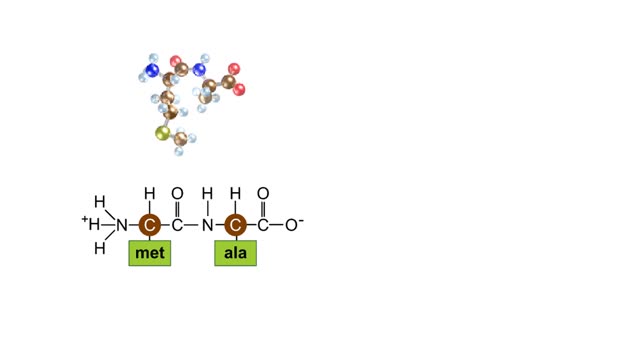
Peptide Bond Formation Animation
By: HWC, Views: 4823
During protein synthesis, peptide bonds link amino acids together in the order specified by DNA instructions. In this case, the first two amino acids in the protein are methionine and alanine. Here are ball-and-stick models of these amino acids. Peptide bond formation is a type of condensatio...
Digestive chemicals - water, gastric acid, bile & bicarbonate
By: HWC, Views: 10788
• Water is the most abundant molecule in ingested fluids. • Water plays a primary role in hydrolytic digestive reactions. • Helps liquefy and transport digestive foodstuffs down the tract. • Transports secretions from accessory digestive organs to gastrointestinal tract. • Aids ...
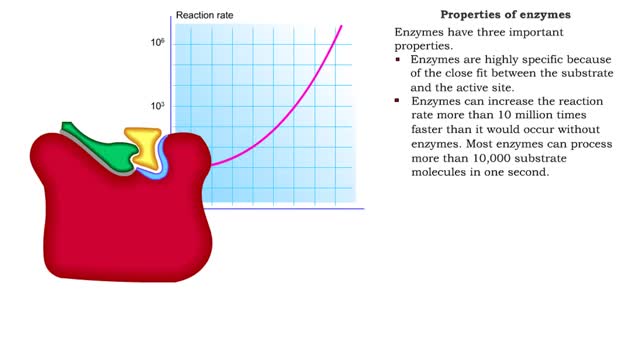
Enzyme structure - Properties of enzymes
By: HWC, Views: 10980
■ Enzymes are proteins that catalyze reactions. ■ Some enzymes have two parts: a protein or apoenzyme and a non-protein or cofactor. ■ Cofactor can be a metal ion or another organic molecule called a coenzyme. ■ Coenzymes often come from vitamins. ■ Cofactors affect the shape of...
Advertisement