Search Results
Results for: 'Na ion and water loss'
By: Administrator, Views: 13689
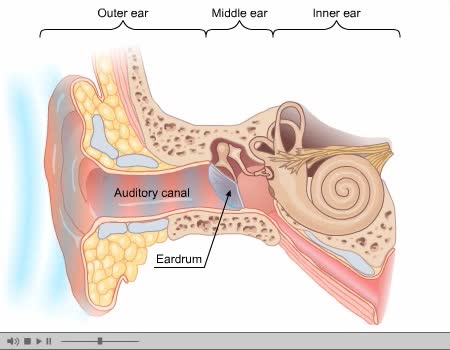
The ear is the organ of hearing and, in mammals, balance. In mammals, the ear is usually described as having three parts—the outer ear, the middle ear and the inner ear. The outer ear consists of the pinna and the ear canal. Since the outer ear is the only visible portion of the ear in most ani...
Primary Active Transport - electrochemical gradient and ion transport / water movement
By: HWC, Views: 10880
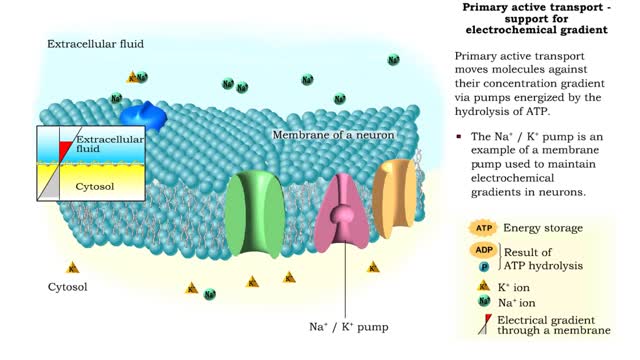
Energy derived from ATP changes the shape of a transporter protein which pumps a substance across a plasma membrane against its concentration gradient An electrochemical gradient is a gradient of electrochemical potential, usually for an ion that can move across a membrane. The gradient consis...
By: HWC, Views: 9457
The slight positive charge of a hydrogen atom in a water molecule can attract an atom with a slight negative charge, such as the nitrogen in a molecule of ammonia. This forms a hydrogen bond between the two atoms. Hydrogen bonds join the two strands of a DNA molecule. Although hydrogen bo...
Factors that increase metabolic rate and heat production
By: HWC, Views: 10817
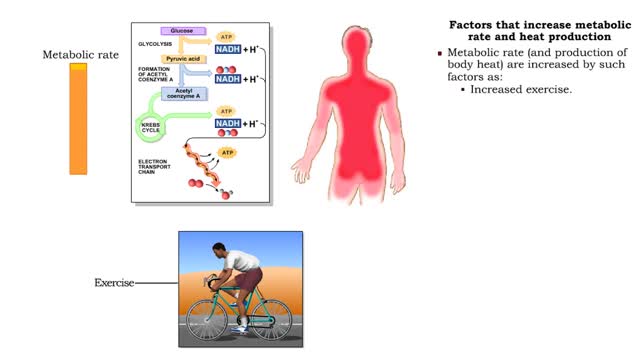
• All vital biochemical reactions are temperature dependent. • The overall rate at which metabolic reactions use energy is known as the metabolic rate. • Metabolic rate greatly determines body temperatures. • Temperature is maintained by balancing the loss of heat to the environment...
By: HWC, Views: 10569
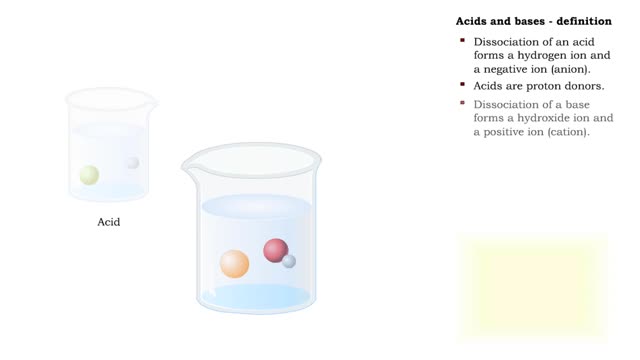
Acids and bases are found all around your house. For example, if you open your pantry or refrigerator, you might see a lot of acids. Fruit juice, soda pop, vinegar, and milk are all examples of acids. The word acid actually comes from a Latin term meaning ''sour.'' Many materials, like sugar for ...
Structures that affect circulation - kidneys and blood volume and skeletal muscle pumping
By: HWC, Views: 11288
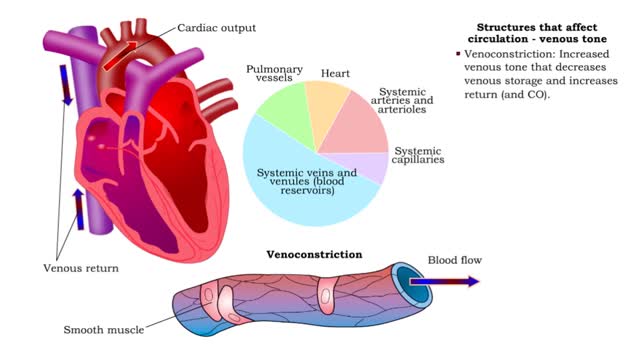
• Kidneys regulate blood volume and blood osmolarity via salt and water reabsorption. • Increased reabsorption increases blood volume and venous return (and CO). • Decreased reabsorption Increases urine production, which decreases blood volume and venous return (and CO). • Systemi...
Bond in biological molecules (Ionic, Covalent and Hydrogen bonds)/ How atoms bond?
By: HWC, Views: 7990
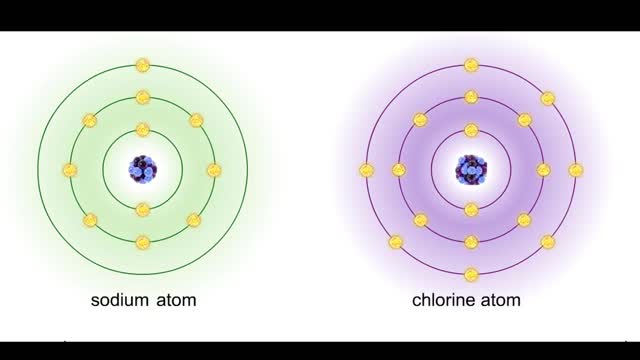
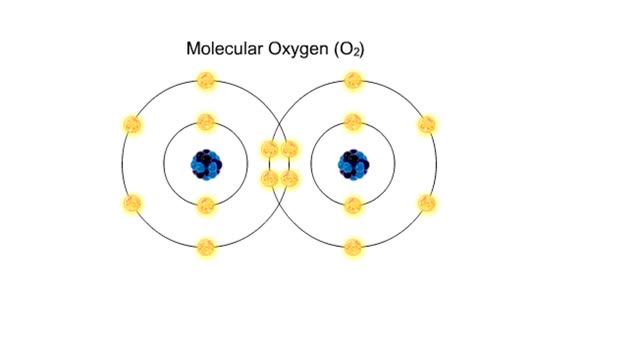
Sodium atoms and chloride atoms have unfilled orbitals in their outer shells. The lone electron in the outermost shell of a sodium atom can be pulled or knocked out. This ionizes the atom. It is now a positively charged sodium ion. A chlorine atom has an electron vacancy in its outer shell and...
ETC Protein Complexes & Chemiosmosis (Total ATP Production and ATP Synthase)
By: HWC, Views: 10389
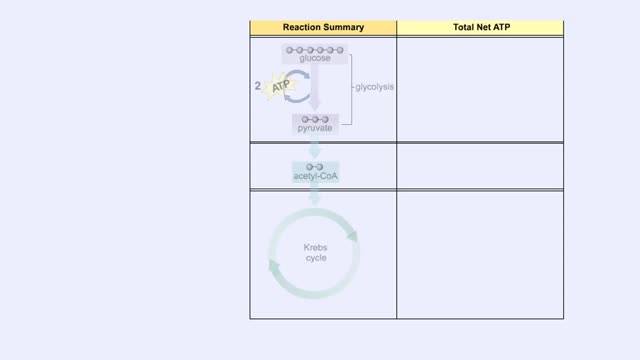
You will notice that FADH2 donates two electrons further downstream than NADH. This results in only two protons being pumped across the inner membrane. The final electron acceptor for these transported electrons is oxygen. Oxygen receives these electrons, plus protons from the aqueous matrix. ...
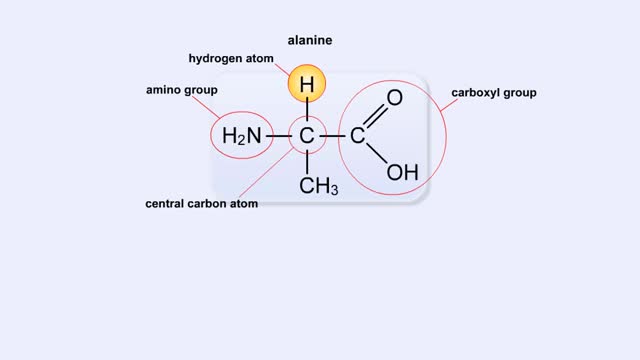
Structure of Amino Acid, Peptide Bonds & Polypeptides
By: HWC, Views: 10296
Here are the molecular formulas of three different amino acids. All amino acids share this backbone. The main difference between every amino acid is the side groups seen here, and these side groups give each of the amino acids their different characteristics. But before we get into that, let's ...
Advertisement