What are Strong & Weak Acids and How they're different?
By: HWC
Date Uploaded: 09/09/2020
Tags: homeworkclinic.com Homework Clinic HWC hydrogen chloride hydronium ions chloride ions acids proton strong acid weak acid hydrochloric acid reversible reaction irreversible reaction acetic acid
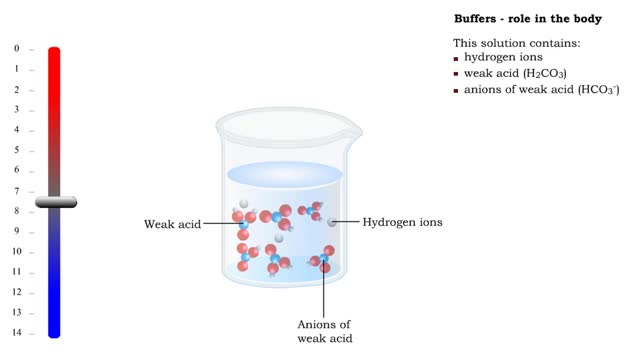
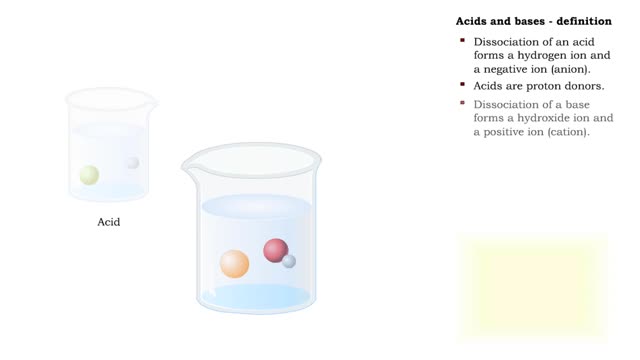
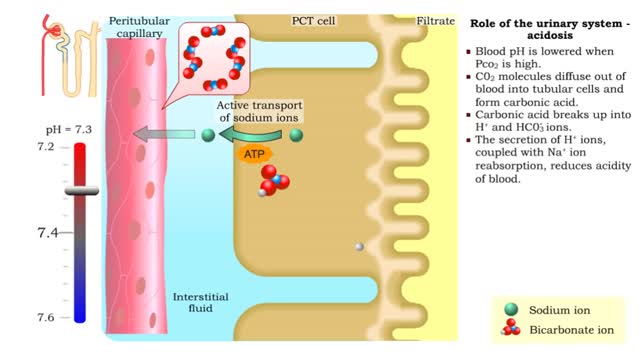
Let's consider the changes that take place when hydrogen chloride, HCI, is added to water. You will need to recognize space-filling models of HCI molecules, hydronium ions (H30+), chloride ions (C11, and water molecules (H20). They are shown at the right. When HC1 molecules dissolve in water, they react with water molecules to form hydronium ions and chloride ions. In this process, an H+ ion (often called a proton) is transferred from each HC1 molecule to a water molecule. When a hydrogen chloride molecule, HC1, meets a water molecule, F170, an H+ ion is transferred from the HC1 to the H70, forming a chloride ion, Cl-, and a hydronium ion, H30+. Now let's take a look at this reaction in solution. The reactants, HCl and H2O, have black outlines, and the products, H30+ and Cl-, will have red outlines. The reactants, HCl and H2O, have black outlines, and the products, H30+ and Cl-, have red outlines. Because the reaction between the HCl molecules and the H2O molecules is not reversible, the final solution contains only H30+ ions, Cl- ions, and water molecules. Because HCl produces hydronium ions, H30+, in solution, it is an acid, and because all of the HCl molecules form H30+ ions, it is a strong acid. The solution of HCl is called hydrochloric acid. The final solution contains H30+ ions, Cl- ions, and water molecules. The HC1 molecules are not present. Because HC1 produces hydronium ions, H30+, in solution, it is an acid, and because all of the HC1 molecules form H30+ ions, it is a strong acid. The solution of HCl is called hydrochloric acid. Now let's consider the changes that take place when acetic acid, HC2H302, is added to water. You will need to recognize space-filling models of molecules, hydronium ions (H30+), acetate ions and water molecules (H20). They are shown at the right. In a change that is similar to the reaction between HC1 and H20, when HC7I-1302 molecules collide with H2O molecules, an H+ ion can be transferred from each HC71-1307 molecule to a H2O molecule, forming a hydronium ion (H30+) and an acetate ion (C2H302-) When an HC2H302 molecule collides with an H2O molecule, an H+ ion can be transferred from the HC2H302 molecule to the H2O molecule, forming a hydronium ion (H30+) and an acetate ion (C7H302-). Unlike the HC1/H70 reaction, the HC2H302/H20 reaction is reversible. When an acetate ion, collides with a hydronium ion, H30+, an H+ can be passed from the H30+ to the C2H302- to form HC2H302 and H2O. When an acetate ion, C2H302-, collides with a hydronium ion, H30+, an H+ can be passed from the H30+ to the C2H302- to form HC2H302- and H2O. Now let's take a look at this reversible reaction in solution. The HC2H302- molecules have a green outline, the H2O molecules that change have black outlines, and the H30+ and C2H302- ions will have red outlines. The HC2H302 molecules have a green outline, the H2O molecules that change have black outlines, and the H30+ and C2H302+ ions have red outlines. Because the reversible reaction favors the uncharged HC2H302 molecules, for every 250 HC2H302 molecules added to water, there are about 249 HC2H302 molecules in the uncharged form, one C7H302- ion, and one H30+ ion. For every 250 HC2H302 molecules added to water, the solution contains about 249 HC2H302 molecules in the uncharged form, one C2H302- ion, and one H30+ ion. The relatively low concentration of the H30+ ions is the reason why acetic acid is classified as a weak acid. Hydrochloric acid is a strong acid because all of the HC1 molecules added to water form H30+ and Cl ions. For every 250 HCl molecules added to water, the solution contains are about 250 Cl ions and 250 H30+ ions.
Add To
You must login to add videos to your playlists.
Advertisement



Comments
0 Comments total
Sign In to post comments.
No comments have been posted for this video yet.