Search Results
Results for: 'Ear'
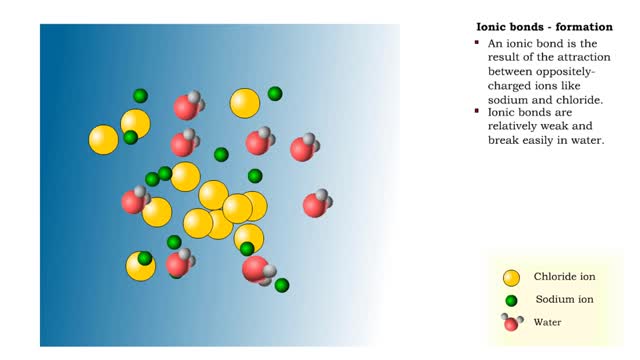
Ionic bonds - role of ions in the body
By: HWC, Views: 11148
Ions • Atoms fill up the outer orbital by transferring electrons from one atom to another. • Atoms now bear a charge and are called ions. • Sodium ion, losing an electron, has a +1 charge. • Chlorine ion, gaining an electron, has a -1 charge. Formation • An ionic bond is t...
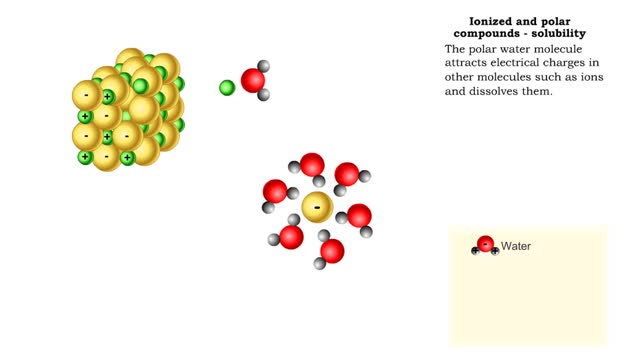
Properties of water -structure of water and polarity (Ionized and polar compounds)
By: HWC, Views: 11037
■ Water transports most of the molecules in the body. ■ The structure of a water molecule allows it to dissolve other molecules. ■ Shared electrons spend more time near the oxygen atom. ■ Oxygen end has a partial negative charge. ■ Hydrogen ends have a partial positive charge....
By: HWC, Views: 10700
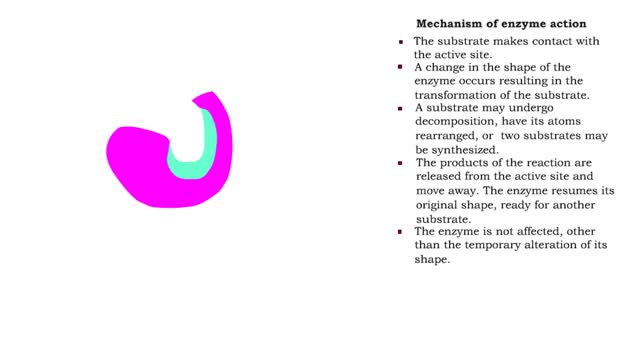
■ The substrate makes contact with the active site. ■ A change in the shape of the enzyme occurs resulting in the transformation of the substrate. ■ A substrate may undergo decomposition, have its atoms rearranged, or two substrates may be synthesized. ■ The products of the reaction...
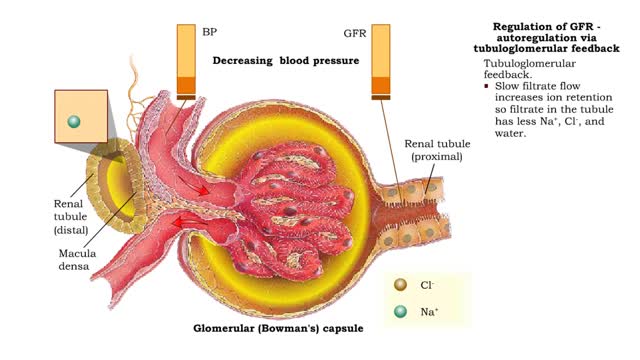
Regulation of GFR: autoregulation via myogenic mechanism Myogenic mechanism
By: HWC, Views: 12467
• GFR can be regulated by adjusting: • Blood flow in and out of the glomerular capillaries. • Surface area of glomerular capillaries. • There are three main ways to make these adjustments: • Renal autoregulation. • Nervous regulation. • Renal autoregulation occurs when...
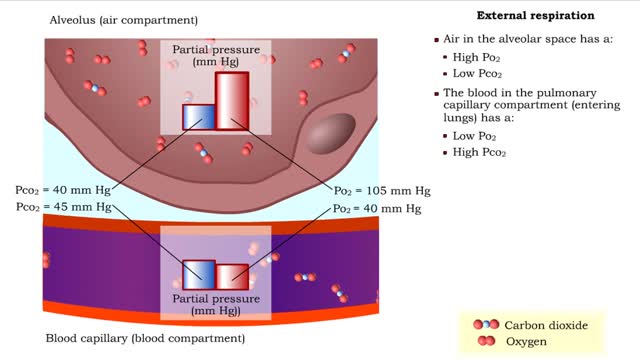
Gas exchange - partial pressure, locations, external and internal respiration
By: HWC, Views: 11064
▪ In a mixture, each individual gas exerts a pressure that is proportional to the concentration of that gas within the mixture. • This part of the total pressure is called a "partial pressure". • A gas moves along the part of the pressure gradient determined by its own concentration. ...
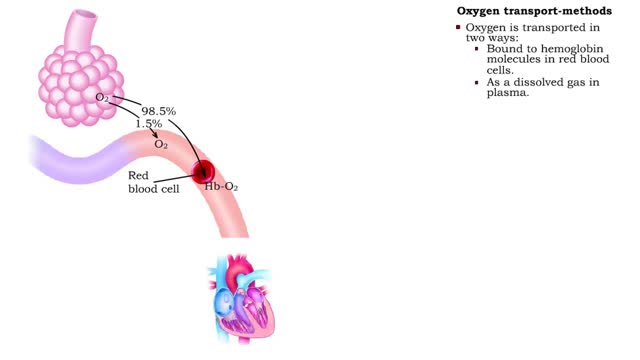
Oxygen transport - methods and oxyhemoglobin
By: HWC, Views: 10766
• The blood is the medium used for gas transport throughout the body. • Oxygen is only available in the lungs. Because the partial pressure of oxygen is higher in the alveoli than in the blood, oxygen diffuses into the blood and is transported to systemic cells. • At the tissues the par...
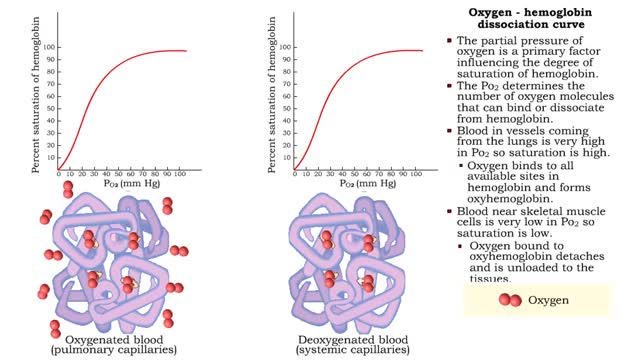
Oxygen - hemoglobin dissociation curve & Hemoglobin's affinity with oxygen - acidity
By: HWC, Views: 11489
• The partial pressure of oxygen is a primary factor influencing the degree of saturation of hemoglobin. • The Po2 determines the number of oxygen molecules that can bind or dissociate from hemoglobin. • Blood in vessels coming from the lungs is very high in Po2 so saturation is high. ...
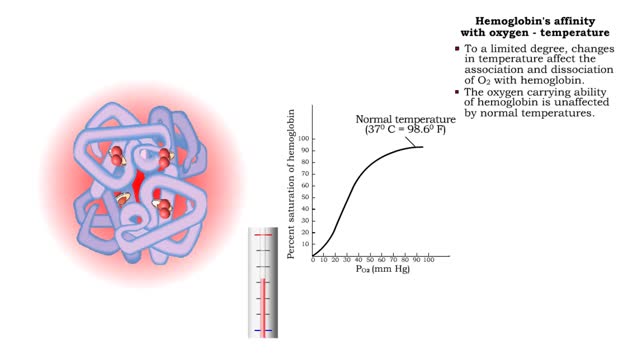
Hemoglobin's affinity with oxygen - carbon dioxide, temperature and bisphosphoglycerate (BPG)
By: HWC, Views: 10880
• The carbon dioxide gas is temporarily converted to carbonic acid in red blood cells by the enzyme carbonic anhydrase, and then further converted to hydrogen and bicarbonate ions. • The result of increased carbon dioxide is decreased pH causing the Bohr effect. • Elevated carbon dioxid...
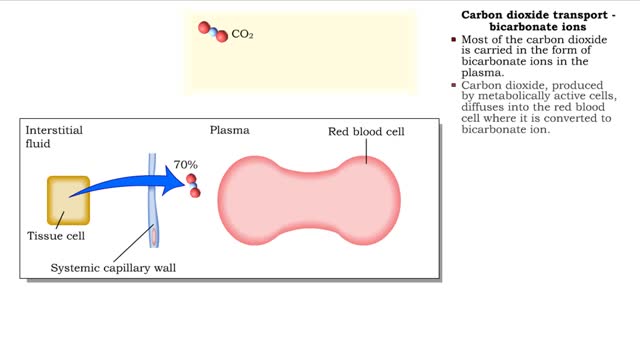
Methods of carbon dioxide transport - carbaminohemoglobin and bicarbonate ions
By: HWC, Views: 10942
• Carbon dioxide is transported three ways: • As bicarbonate ions in the plasma. • Bound to hemoglobin. • As a dissolved gas in the plasma. • A small percent of carbon dioxide is transported as a dissolved gas. • Some of the carbon dioxide is bound to hemoglobin, in the fo...
Advertisement