Search Results
Results for: 'The pH scale • Expresses concentration of H . • range: 0-14. • 7 is neutral. • Less 7 is acid. • greater 7 is basic (alkaline). Strong acids - role in the body ■ In strong acids all molecules dissociate. ■ HC1 is highly acidic and found only in the stomach. • HC1 in gastric juice is important for the digestion of proteins. • HCl kills bacteria in food by destroying their proteins. Weak acids - role in the body ■ Carbonic acid is produced from CO2 and H2O. ■ H2CO3'
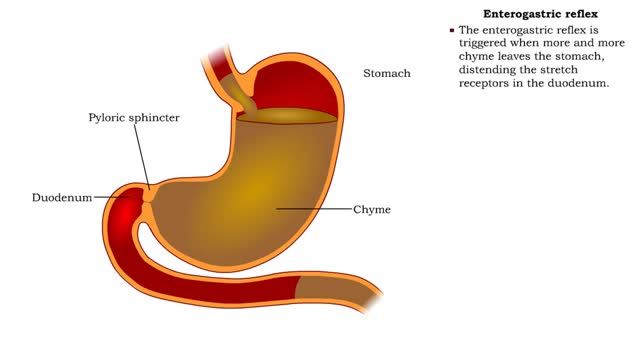
Stomach peristalsis & Enterogastric reflex
By: HWC, Views: 6082
• Food enters, distending the stomach. • Stretch receptors activate enteric reflexes that promote peristaltic movements. • These movements, called mixing waves, begin to mix the food with stomach secretions. • Mixing waves force the digesting food (chyme) toward and through the pylo...
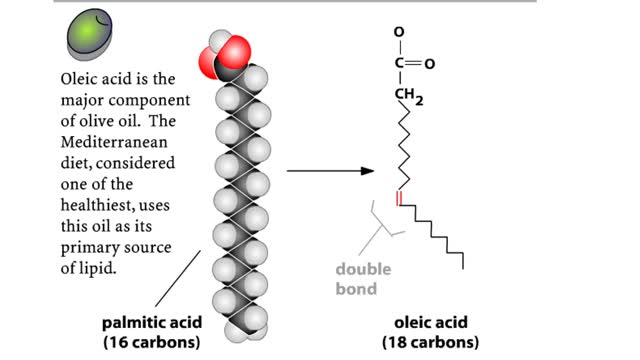
Major Elements in Biological Molecules: Lipids
By: HWC, Views: 5934
A triglyceride (also called triacylglycerol) is composed of three fatty acid molecules and one glycerol molecule. The fatty acids attach to the glycerol molecule by a covalent ester bond. The long hydrocarbon chain of each fatty acid makes the triglyceride molecule nonpolar and hydrophobic. Pa...
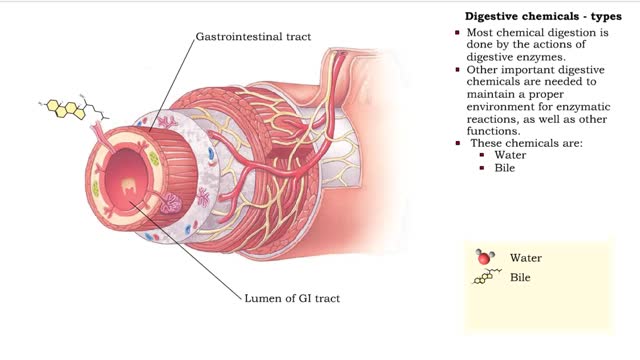
Digestive chemicals - types & enzymes
By: HWC, Views: 6631
• Chemical digestion breaks down food as it moves through the digestive tract. • Using enzymes and other digestive chemicals, the process reduces food particles into nutrient molecules that can be absorbed. • Most chemical digestion is done by the actions of digestive enzymes. • O...
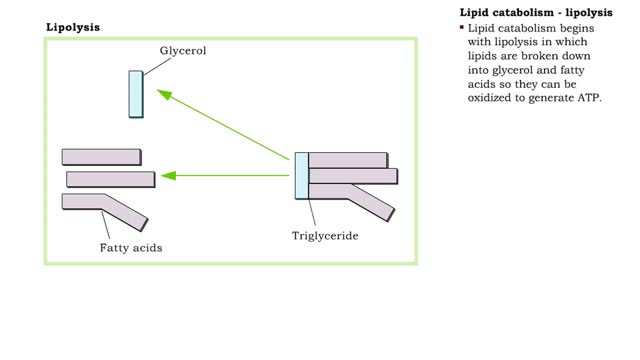
Lipid catabolism - lipolysis and beta oxidation and oxidation of fatty acids
By: HWC, Views: 6925
• Digestion hydrolyzes lipids into fatty acids and glycerol. • Fatty acids and glycerol are: • Oxidized to generate ATP. • Used to produce triglycerides that are stored as energy reserves in adipose tissue. • Lipid catabolism begins with lipolysis in which lipids are broken do...
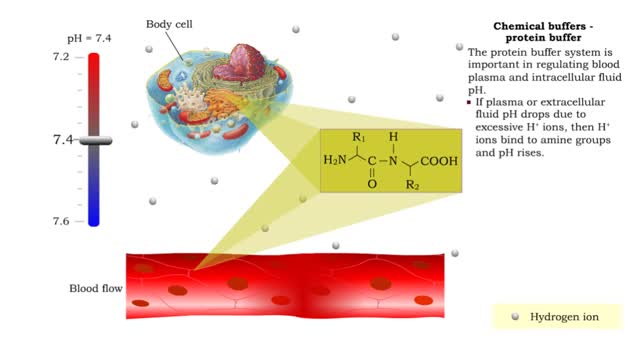
Chemical Buffers - protein buffer, phosphate buffer system and bicarbonate buffer system
By: HWC, Views: 6885
• There are a variety of chemicals in body fluids that prevent the fluids from undergoing large changes in. • These chemicals buffer or regulate fluctuations in H+ concentration. • Chemical buffers: • Bind to H+ ions when there are too many in a solution so pH remains normal. •...
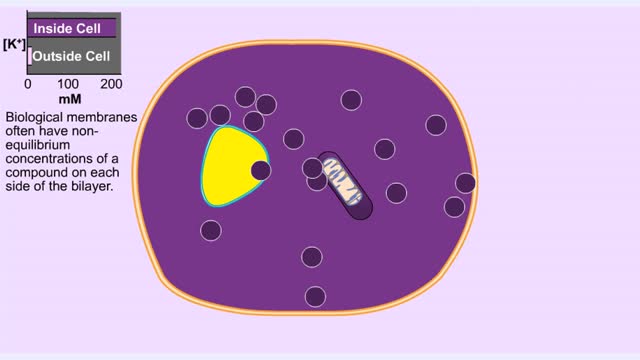
Membrane Protein and Facilitated Transport (Passive Vs Active)
By: HWC, Views: 6271
Membrane proteins are common proteins that are part of, or interact with, biological membranes. Membrane proteins fall into several broad categories depending on their location. Integral membrane proteins span the membrane, with hydrophobic amino acids interacting with the lipid bilayer and hy...
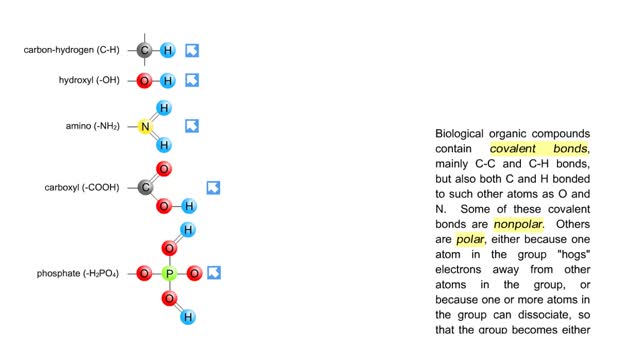
By: HWC, Views: 6086
Biological organic compounds contain covalent bonds, mainly C-C and C-H bonds, but also both C and H bonded to such other atoms as O and N. Some of these covalent bonds are nonpolar. Others are polar, either because one atom in the group "hogs" electrons away from other atoms in the group, or...
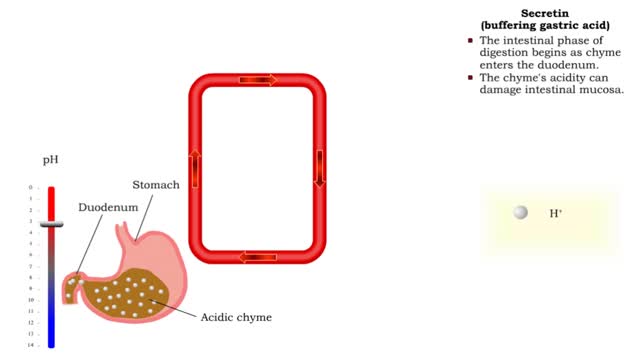
Gastrin (gastric emptying) & Secretin (buffering gastric acid)
By: HWC, Views: 6223
• Gastrin also binds to the smooth muscle cells in the stomach causing: • Increased gastric motility. • Opening of pyloric sphincter. • Increased gastric emptying. • The intestinal phase of digestion begins as chyme enters the duodenum. • The chyme's acidity can damage int...
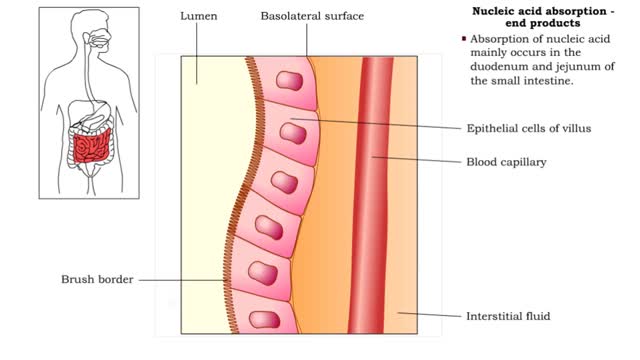
Nucleic acid digestion - brush border enzymes, end products & transport mechanism
By: HWC, Views: 6447
• Further digestion occurs at the microvilli (brush border) of the epithelial cells of the villi in the small intestine. • Two brush border enzymes complete nucleic acid digestion: • Phosphatases, which catalyze the cleavage of a phosphate to form a nucleoside (nitrogenous base and pent...
Advertisement