Search Results
Results for: 'The pH scale • Expresses concentration of H . • range: 0-14. • 7 is neutral. • Less 7 is acid. • greater 7 is basic (alkaline). Strong acids - role in the body ■ In strong acids all molecules dissociate. ■ HC1 is highly acidic and found only in the stomach. • HC1 in gastric juice is important for the digestion of proteins. • HCl kills bacteria in food by destroying their proteins. Weak acids - role in the body ■ Carbonic acid is produced from CO2 and H2O. ■ H2CO3'
By: HWC, Views: 861
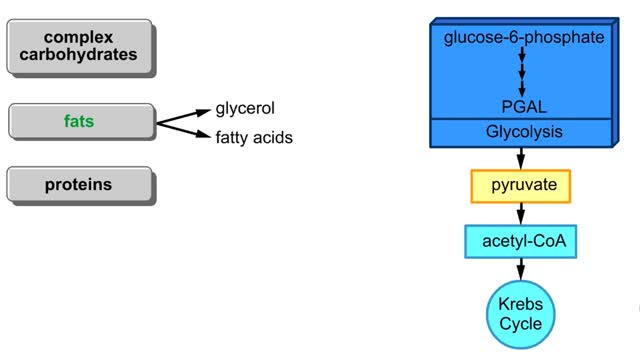
Points at which organic compounds enter the reaction stages of aerobic respiration. Complex carbohydrates are broken down into simple sugars, such as glucose. They become the substrates for glycolysis. If your body doesn't need to burn glucose for energy, glucose-6-phosphate can be co...
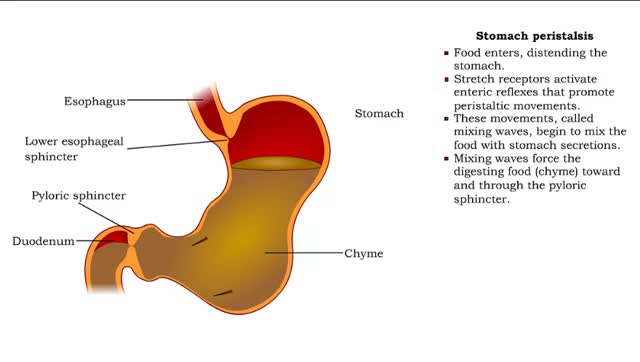
Stomach peristalsis - Movement of Food Through the Small Intestine
By: HWC, Views: 6688
Peristalsis is a series of wave-like muscle contractions that moves food to different processing stations in the digestive tract. The process of peristalsis begins in the esophagus when a bolus of food is swallowed. The strong wave-like motions of the smooth muscle in the esophagus carry the food...
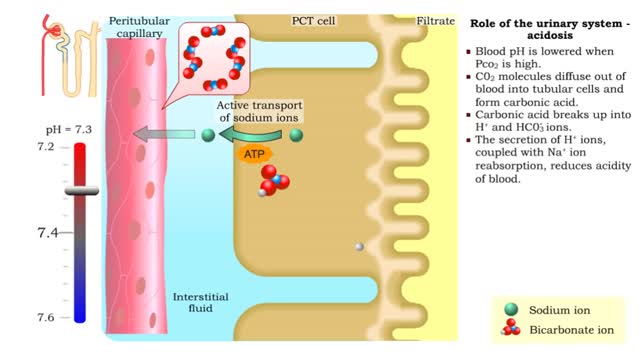
Role of the urinary system - acidosis and alkalosis
By: HWC, Views: 6886
• Tubular cells of the proximal convoluted tubule and collecting tubules can alter filtrate pH and therefore blood pH. • These cells can affect blood pH with two coupled mechanisms: • Reabsorption of bicarbonate ions. • Secretion of hydrogen ions. • The reabsorption of bicarbonate...
By: HWC, Views: 6901
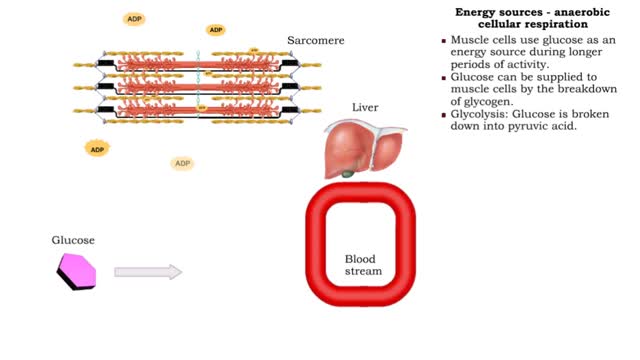
• The amount of ATP stored in a skeletal muscle cell can only provide muscular activity for two to three seconds. • Muscle cells must be able to generate additional molecules of ATP to continue contracting. • Muscle cells can generate ATP from several processes: • Phosphogen syste...
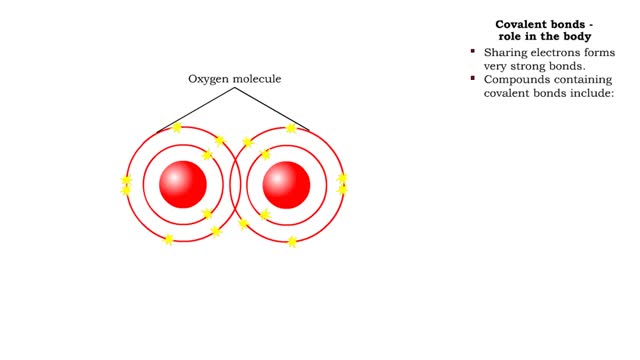
Covalent bonds - role in the body
By: HWC, Views: 6667
A covalent bond is formed when atoms share one or more pairs of electrons. This is opposed to an ionic bond, where electrons are actually transferred from one atom to another. Formation • Atoms fill up the outer orbital by sharing electrons. • Two oxygen atoms sharing electrons form on...
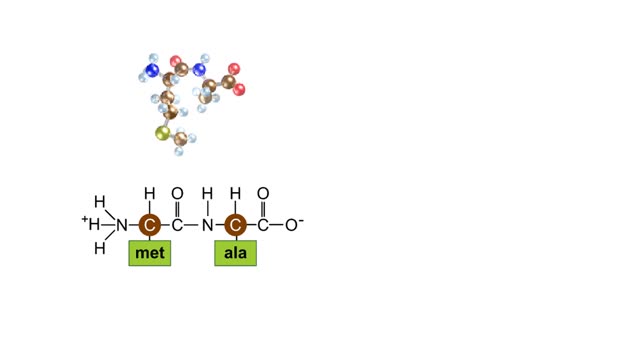
Peptide Bond Formation Animation
By: HWC, Views: 513
During protein synthesis, peptide bonds link amino acids together in the order specified by DNA instructions. In this case, the first two amino acids in the protein are methionine and alanine. Here are ball-and-stick models of these amino acids. Peptide bond formation is a type of condensatio...
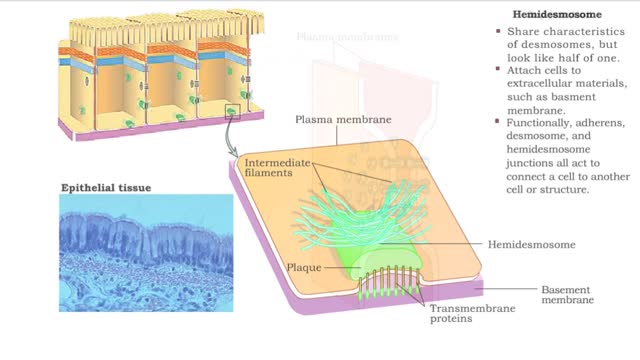
Type of Cell Junctions - Desmosome, Hemidesmosomes and Gap Junctions
By: HWC, Views: 7020
Cell Junctions: Cell junctions are found in some multi-cellular organisms. They exist of complexes and are found between cells and between cells and other structures. The junctions provide a way for cells to connect and exchange signals. What are tight junctions, desmosomes, and gap junctions...
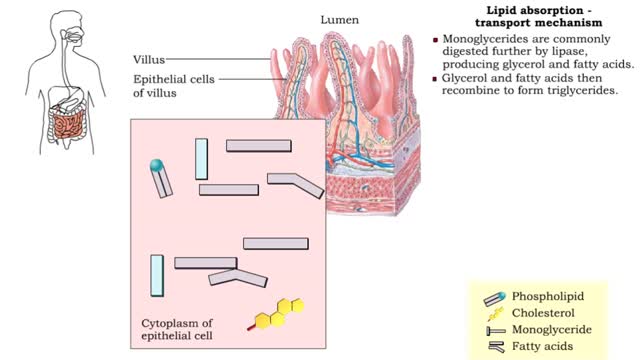
Lipid absorption - end products & transport mechanism
By: HWC, Views: 6200
• The end products, fatty acids and monoglycerides, depend on bile salts for absorption. • Bile salts form micelles (tiny spheres), which ferry fatty acids and monoglycerides to epithelial cells. • Free fatty acids, monoglycerides, and some phospholipids and cholesterol molecules, dif...
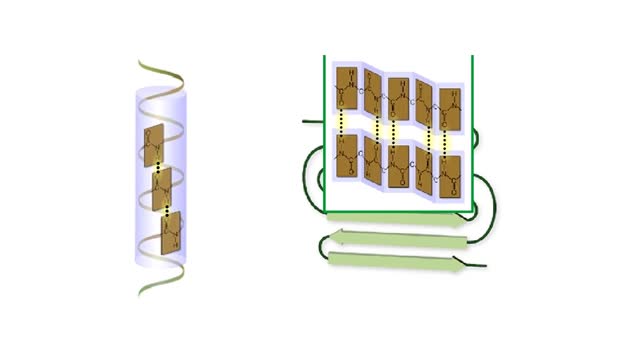
Secondary and tertiary levels of protein structure Animation
By: HWC, Views: 565
Amino acid sequence dictates a protein's final shape. The presence of certain amino acids favors a pattern of hydrogen bonding that causes part of the polypeptide chain to coil and twist into an alpha helix. The presence of other amino acids enables hydrogen bonding between strand like re...
Advertisement