Search Results
Results for: 'amino acid-based food additives'
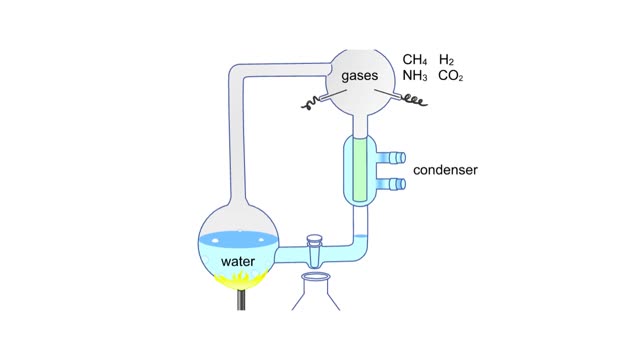
Miller's reaction chamber experiment Animation
By: HWC, Views: 4732
A simple diagram of Stanley Miller and Harold Urey's experimental apparatus. The lower portion of the apparatus was filled with water. The upper portion was filled with a mixture of gases that simulated the earth's early atmosphere. Examples are methane, ammonia, hydrogen and carbon dioxide. ...
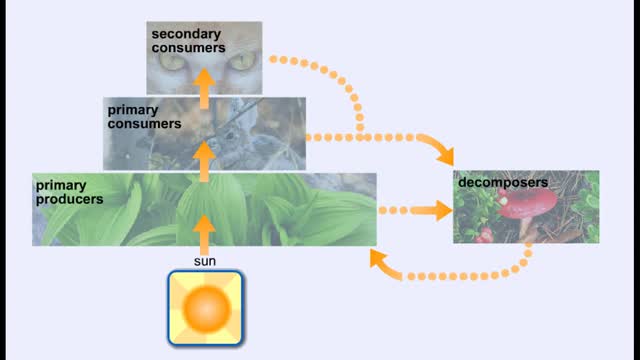
Energy Flow - Trophic Levels and Food
By: HWC, Views: 10640
All of these relationships between different species are founded on one thing: energy. Organisms get food in order to get energy, which is used by the organism for growth, maintaining health, and reproduction. We can classify the members of a community according to how they obtain food. Produc...
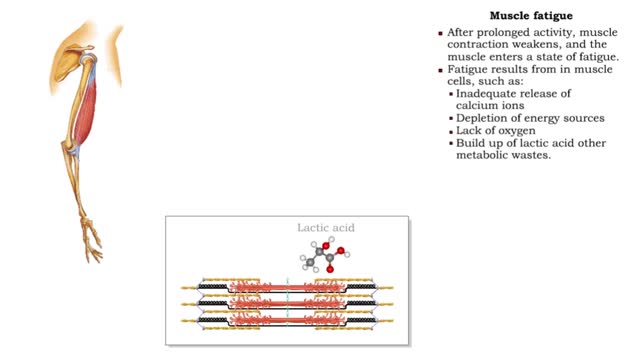
By: HWC, Views: 10749
• After prolonged activity, muscle contraction weakens, and the muscle enters a state of fatigue. • Fatigue results from in muscle cells, such as: • Inadequate release of calcium ions • Depletion of energy sources • Lack of oxygen • Build up of lactic acid other metabolic w...
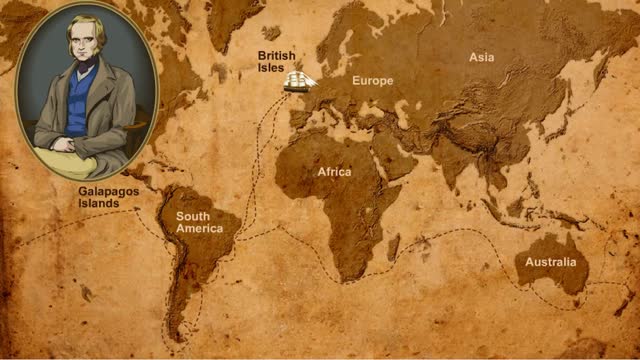
Diversity of Living Creatures - Charles Darwin & the Beagle's Voyage
By: HWC, Views: 10619
As we look around at the living creatures on Earth, we see a great amount of diversity. In some cases, the differences are quite noticeable, like the differences between plants and animals. Some differences are not as easy to see, like the differences between two species of lizard. How did all...
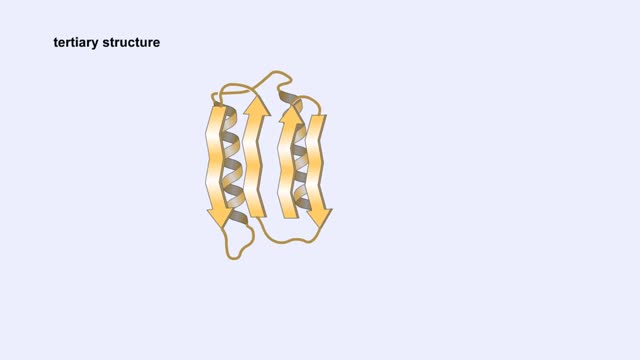
Protein Structure - Primary, Secondary, Tertiary and Quaternary
By: HWC, Views: 10934
A protein's first order structure, or primary structure, begins with the amino acid sequence of the polypeptide chain. The 20 different amino acids can be arranged in an infinite number of sequences. For example, the hormone insulin, which regulates the uptake of glucose from the blood into ce...
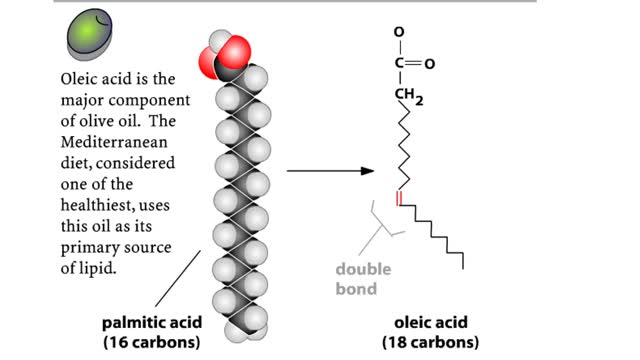
Major Elements in Biological Molecules: Lipids
By: HWC, Views: 10416
A triglyceride (also called triacylglycerol) is composed of three fatty acid molecules and one glycerol molecule. The fatty acids attach to the glycerol molecule by a covalent ester bond. The long hydrocarbon chain of each fatty acid makes the triglyceride molecule nonpolar and hydrophobic. Pa...
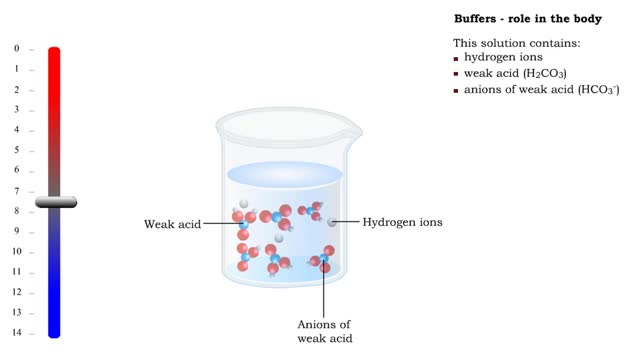
Buffers definition and the role of buffer in the body
By: HWC, Views: 11053
■ Too many H+ break hydrogen bonds and a protein comes apart. ■ Buffers react with excess H+ to protect proteins from breaking down. ■ Buffers consist of weak acid plus anions of that weak acid. This solution contains: • hydrogen ions • weak acid (H2CO3) • anions of we...
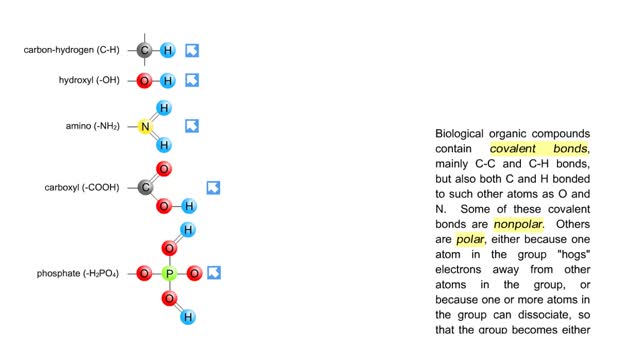
By: HWC, Views: 10530
Biological organic compounds contain covalent bonds, mainly C-C and C-H bonds, but also both C and H bonded to such other atoms as O and N. Some of these covalent bonds are nonpolar. Others are polar, either because one atom in the group "hogs" electrons away from other atoms in the group, or...
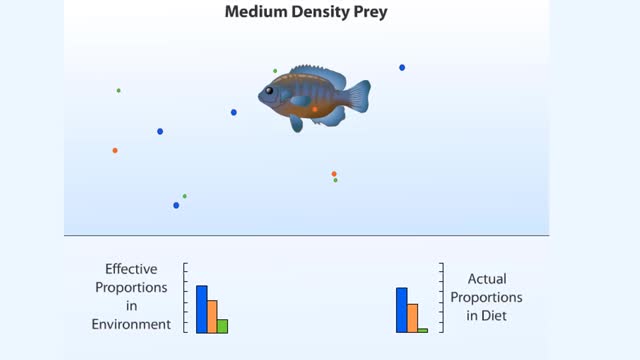
How does an animal choose what food to eat?
By: HWC, Views: 10493
One might assume that natural selection has influenced the foraging behaviors of animals, and that most animals forage efficiently, spending the least energy to gain the most nutrients. This is the underlying assumption of optimality modeling, a scientific approach to studying foraging behavior. ...
Advertisement