Search Results
Results for: 'molecule of ammonia'
Glycolysis - Introduction to ATP and the burning of sugar
By: HWC, Views: 11970
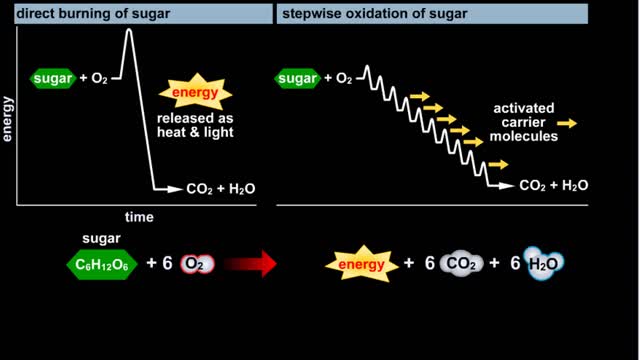
Do you use sugar with your coffee or tea? Or do you occasionally drink a sport or soft drink? As millions of people do each day, they obtain energy from the sugar added or contained in these drinks. How can we understand this concept of energy within a sugar molecule? Let's take a tablespoon ...
By: HWC, Views: 11957
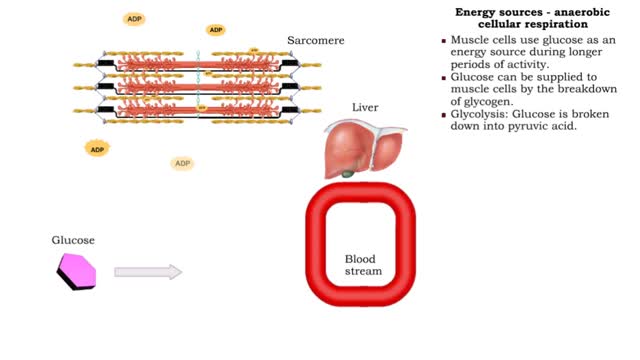
• The amount of ATP stored in a skeletal muscle cell can only provide muscular activity for two to three seconds. • Muscle cells must be able to generate additional molecules of ATP to continue contracting. • Muscle cells can generate ATP from several processes: • Phosphogen syste...
Non-polar compounds - insolubility
By: HWC, Views: 11817
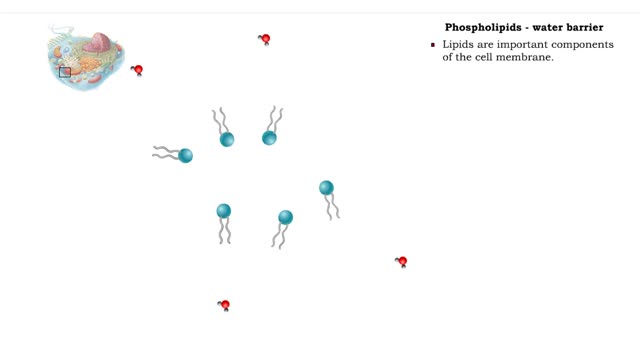
• A non-polar molecule has uniform distribution of electrons. • Non-polar compounds like fatty acids in lipids have a high proportion of carbon and hydrogen. • Lipids possess no charge or partial charge. • Lipids are not attracted to water molecules. • Lipids are not soluble in...
Chloroplast Structure & Light Dependent Reactions (Photosystem 1 and 2 Cyclic Electron Flow)
By: HWC, Views: 11289
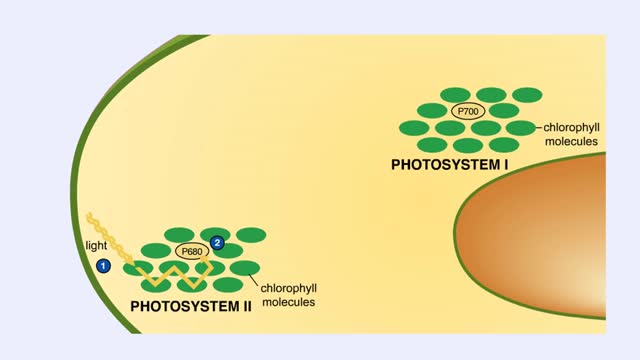
The leaf is the principle photosynthetic organ of the plant. This is a cross section of a leaf. The rectangular-shaped cells are part of the photosynthetic tissue called the palisade mesophyll. Each photosynthetic cell can contain several hundred organelles known as chloroplasts. The chlorop...
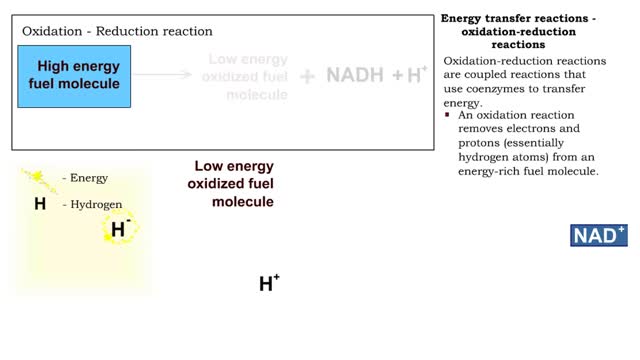
Types of energy transfer reactions: oxidation-reduction reactions and ATP generation reactions
By: HWC, Views: 12343
■ Metabolism balances anabolic and catabolic reactions. ■ Anabolism is energy transfer from ATP to simpler molecules in order to build them up into larger, more complex molecules. ■ Catabolism is breaking down larger, more complex molecules, usually to transfer energy from them in order...
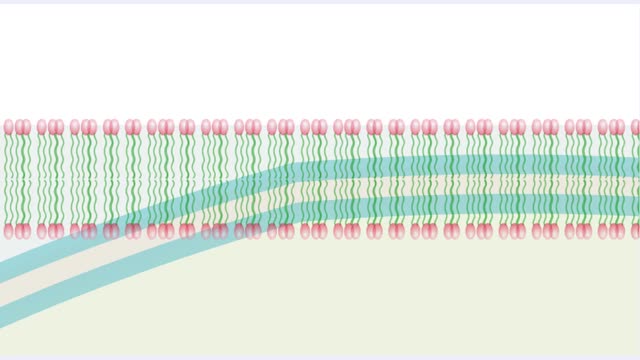
Molecules, Membrane Permeability and Structure
By: HWC, Views: 11186
Organisms are not isolated system at equilibrium and need to intake nutrients and electrolytes as remove wastes. Similarly Cells within an organism must also exchange compound by passing them through membrane. The permeability of a membrane is the rate of passive diffusion of molecules th...
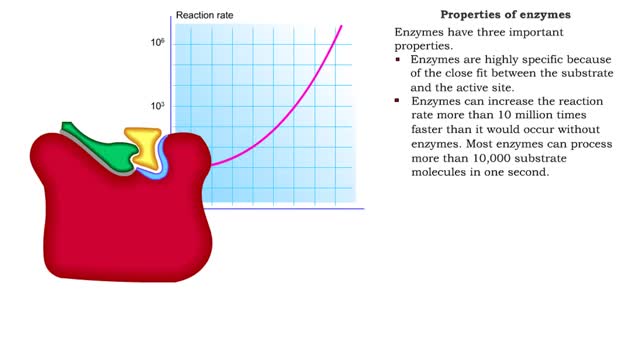
Enzyme structure - Properties of enzymes
By: HWC, Views: 11691
■ Enzymes are proteins that catalyze reactions. ■ Some enzymes have two parts: a protein or apoenzyme and a non-protein or cofactor. ■ Cofactor can be a metal ion or another organic molecule called a coenzyme. ■ Coenzymes often come from vitamins. ■ Cofactors affect the shape of...
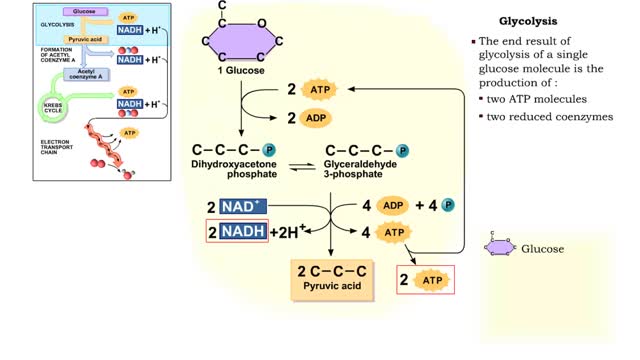
By: HWC, Views: 12002
The first reactions involve a single 6-carbon glucose sugar undergoing phosphorylation using two ATP molecules and resulting in two 3-carbon compounds. • The rest of this pathway involves an oxidation reduction reaction, forming two reduced coenzymes, and generation of four ATP molecules. ...
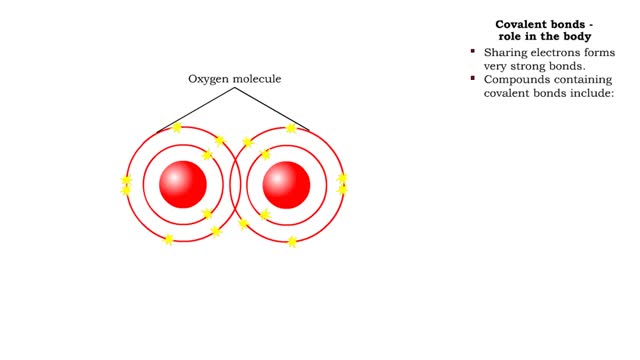
Covalent bonds - role in the body
By: HWC, Views: 11686
A covalent bond is formed when atoms share one or more pairs of electrons. This is opposed to an ionic bond, where electrons are actually transferred from one atom to another. Formation • Atoms fill up the outer orbital by sharing electrons. • Two oxygen atoms sharing electrons form on...
Advertisement