Search Results
Results for: 'polymerase chain reaction'
Enzyme structure - Properties of enzymes
By: HWC, Views: 11687
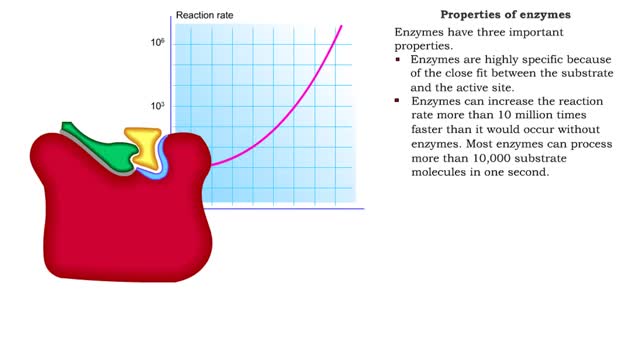
■ Enzymes are proteins that catalyze reactions. ■ Some enzymes have two parts: a protein or apoenzyme and a non-protein or cofactor. ■ Cofactor can be a metal ion or another organic molecule called a coenzyme. ■ Coenzymes often come from vitamins. ■ Cofactors affect the shape of...
Krebs cycle : Formation of acetyl coenzyme A and Electron transport chain
By: HWC, Views: 11989
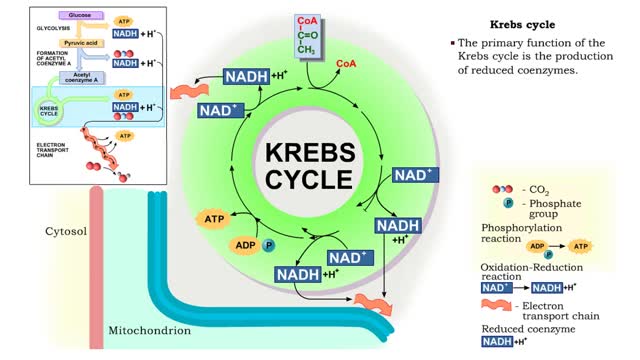
The oxidation of glucose to produce ATP is cellular respiration. Four sets of reactions are involved: Glycolysis Formation of acetyl coenzyme A Krebs cycle reactions Electron transport chain reactions • The second pathway of glucose catabolism, formation of acetyl coenzyme A, is a transi...
Subunits of DNA And Semi Conservative Replication
By: HWC, Views: 8041
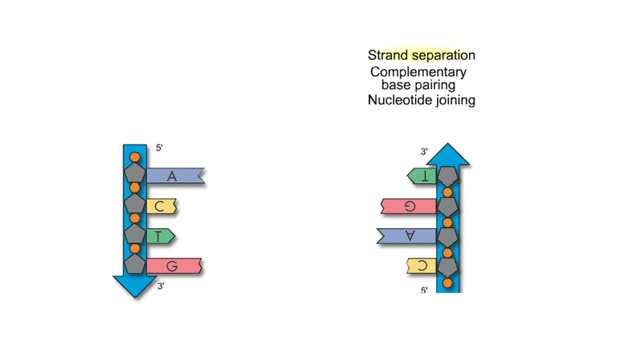
Adenine is a purine with a double-ring structure. In double-stranded DNA, adenine base-pairs with thymine. Guanine is a purine with a double-ring structure. In double-stranded DNA, guanine base-pairs with cytosine. Thymine is a pyrimidine with a single-ring structure. In double-stranded DNA, th...
By: HWC, Views: 11998
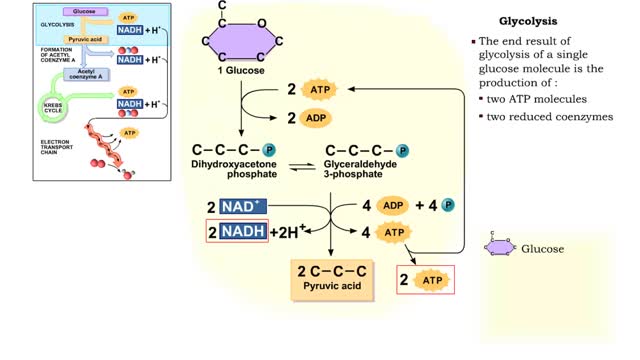
The first reactions involve a single 6-carbon glucose sugar undergoing phosphorylation using two ATP molecules and resulting in two 3-carbon compounds. • The rest of this pathway involves an oxidation reduction reaction, forming two reduced coenzymes, and generation of four ATP molecules. ...
How proteins function? How do proteins work?
By: HWC, Views: 11412
How proteins function is really about how proteins "do work" in cells. How do proteins work? Let's start thinking about protein function by looking at something important to you: your hair. Keratin is a structural protein that is composed of 2 intertwined or helical strands. Keratin is also f...
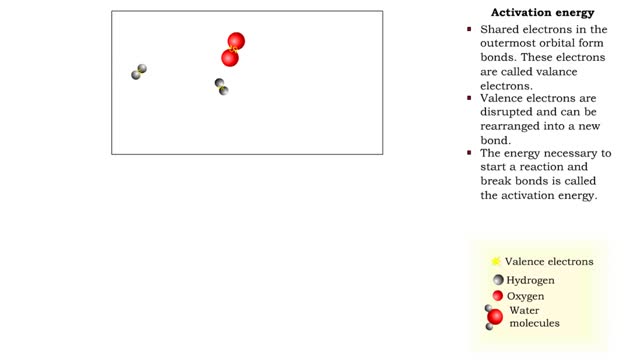
Activation Energy - Valence Electrons
By: HWC, Views: 11191
■ Shared electrons in the outermost orbital form bonds. These electrons are called valence electrons. ■ Valence electrons are disrupted and can be rearranged into a new bond. ■ The energy necessary to start a reaction and break bonds is called the activation energy. ■ Reactants have...
By: HWC, Views: 11177
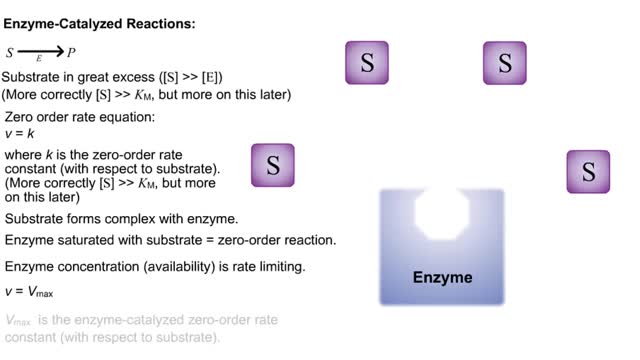
S P Substrate in great excess ([S] -- [E]) (More correctly [S] -- KM, but more on this later) Zero order rate equation: v = k where k is the zero-order rate constant (with respect to substrate). (More correctly [S] -- KM, but more on this later) Substrate forms complex with enzyme. ...
By: HWC, Views: 11537
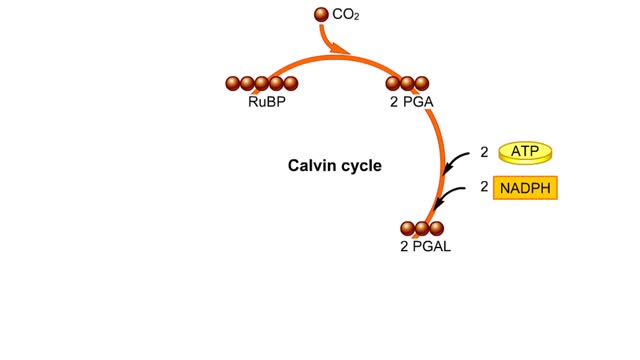
he light-independent reactions make sugars by way of a cyclic pathway called the Calvin cycle. The cycle begins when rubisco attaches a carbon from carbon dioxide to ribulose bisphosphate. The molecule that forms splits into two molecules of PGA. Each PGA gets a phosphate group from ATP a...
By: HWC, Views: 11553
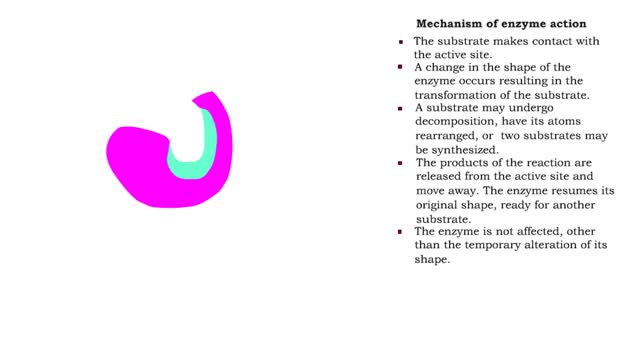
■ The substrate makes contact with the active site. ■ A change in the shape of the enzyme occurs resulting in the transformation of the substrate. ■ A substrate may undergo decomposition, have its atoms rearranged, or two substrates may be synthesized. ■ The products of the reaction...
Advertisement