Protein Structure - Primary, Secondary, Tertiary and Quaternary
By: HWC
Date Uploaded: 03/10/2020
Tags: homeworkclinic.com Homework Clinic HWC Protein Structure polypeptide chain hormone insulin endoplasmic reticulum Golgi apparatus Frederick Sanger alpha helix noncovalent interactions covalent bonds hydrogen bonds ionic bonds hydrophobic interactions van der Waals forces hemoglobin collagen globular proteins wrinkled skin
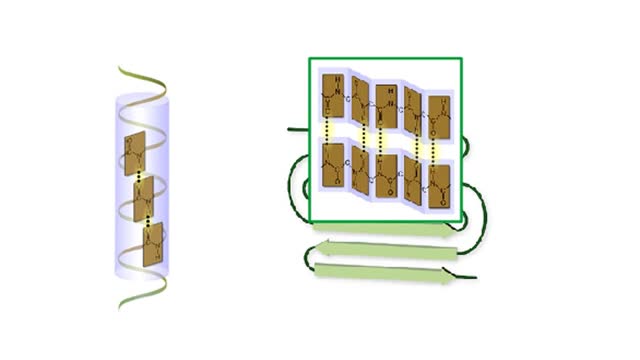
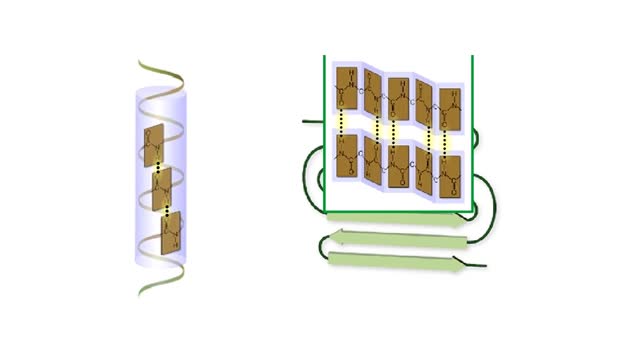
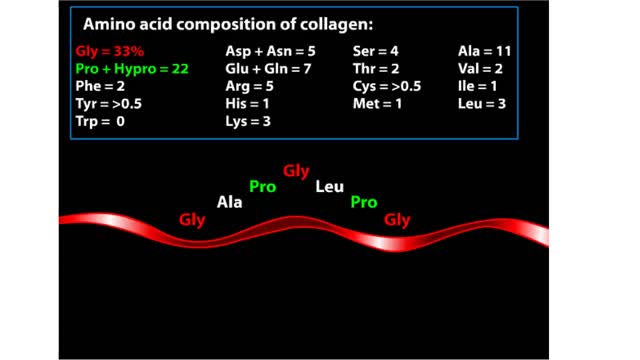
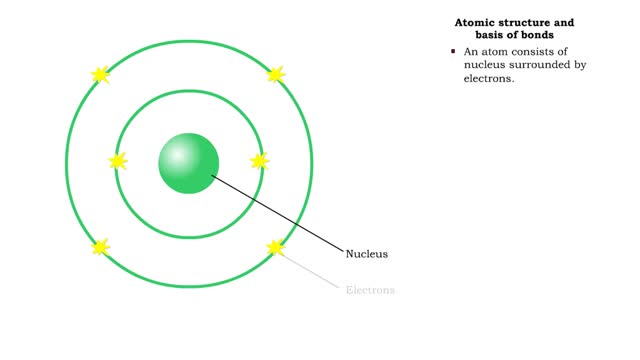
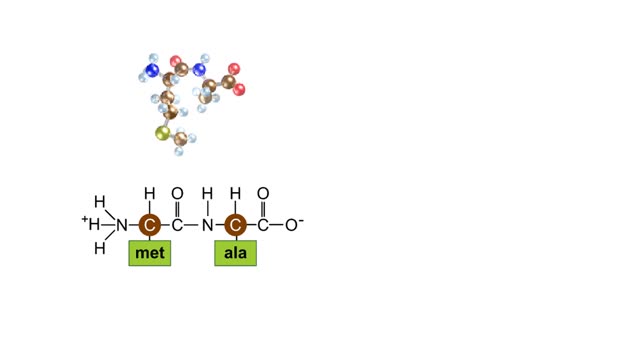
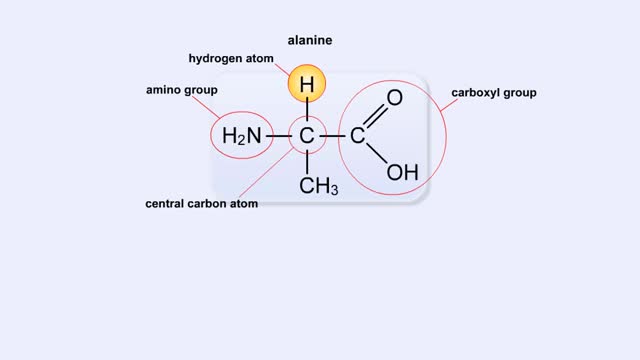
A protein's first order structure, or primary structure, begins with the amino acid sequence of the polypeptide chain. The 20 different amino acids can be arranged in an infinite number of sequences. For example, the hormone insulin, which regulates the uptake of glucose from the blood into cells, consists of 2 polypeptide chains (the alpha and beta chains) consisting of 21 and 30 amino acids, respectively. An intervening connecting peptide chain, the C-peptide, initially unites the alpha and beta chains. A signal peptide chain targets the protein to the endoplasmic reticulum, where it is further processed before being sent on to the Golgi apparatus where packaging occurs prior to export from the cell. Insulin was the first protein whose primary sequence (structure) was determined by Frederick Sanger in 1953. Based on the nature of the side groups of amino acids, polypeptides exhibit regions of the chain that assume a helical arrangement or a pleated sheet arrangement. These arrangements, also called domains, are referred to as the secondary structure. The helical secondary structure is called an alpha helix. The helical shape is maintained by hydrogen bonds, as shown here. The pleated sheet secondary structure is called a beta sheet. Beta sheets are formed by the looping of the polypeptide chain back on itself. Adjacent chains of a beta sheet are held together by hydrogen bonds. The final folded three-dimensional shape of a polypeptide chain with its helices and pleated sheets is referred to as the protein's tertiary structure. Here the polypeptide has several domains of helices and pleated sheets. The chemical nature of the side groups will determine the types of interactions between the regions of the folded polypeptide. These interactions can be either covalent bonds (in the form of disulfide bonds) or noncovalent interactions such as hydrogen bonds, ionic bonds, hydrophobic interactions, and van der Waals forces. When two or more polypeptide chains participate in the final shape of a protein, the resulting structure is called the quaternary structure. Chemical interactions between the side groups of the polypeptide chains maintain the 3-dimensional structure of the protein. Examples of proteins with quaternary structure include hemoglobin and collagen. Hemoglobin is an example of a protein that exhibits quaternary structure. It consists of two identical alpha chains and two identical beta chains. These chains are referred to as subunits. Each of the four polypeptide chains possesses primary, secondary, and tertiary structure. When the shape of the protein is more or less spherical, as is the case with hemoglobin, these proteins are called globular proteins. Collagen is an example of a fibrous protein. Fibrous proteins form long strands either from a single polypeptide chain or when two or three polypeptide chains wrap around each other in a helical pattern. This arrangement gives collagen great strength. Accounting for nearly 40% of all protein in the body, collagen is present in connective tissues where it supplies strength and flexibility. The aging process reduces the amount of collagen and wrinkles and lines appear on the face as a response. Collagen injections are a frequent cosmetic solution to wrinkled skin.
Add To
You must login to add videos to your playlists.
Advertisement




Comments
0 Comments total
Sign In to post comments.
No comments have been posted for this video yet.