Search Results
Results for: 'electrons orbit'
Hydrogen bonds - role in the body
By: HWC, Views: 11299
A hydrogen bond is the electromagnetic attraction between polar molecules in which hydrogen is bound to a larger atom, such as oxygen or nitrogen. This is not a sharing of electrons, as in a covalent bond. Instead, this is an attraction between the positive and negative poles of charged atoms. ...
By: HWC, Views: 5212
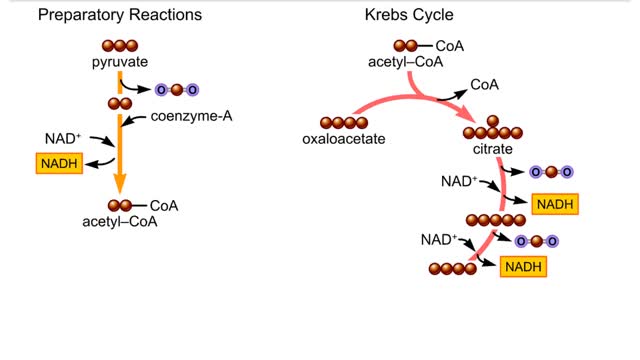
The second-stage reactions of aerobic respiration. The second-stage reactions occur in a mitochondrion's inner compartment. In the first preparatory reaction, a carbon atom is stripped from pyruvate and released as carbon dioxide. The remaining carbons combine with coenzyme A and give ...
Bond in biological molecules (Ionic, Covalent and Hydrogen bonds)/ How atoms bond?
By: HWC, Views: 8311
Sodium atoms and chloride atoms have unfilled orbitals in their outer shells. The lone electron in the outermost shell of a sodium atom can be pulled or knocked out. This ionizes the atom. It is now a positively charged sodium ion. A chlorine atom has an electron vacancy in its outer shell and...
By: HWC, Views: 4881
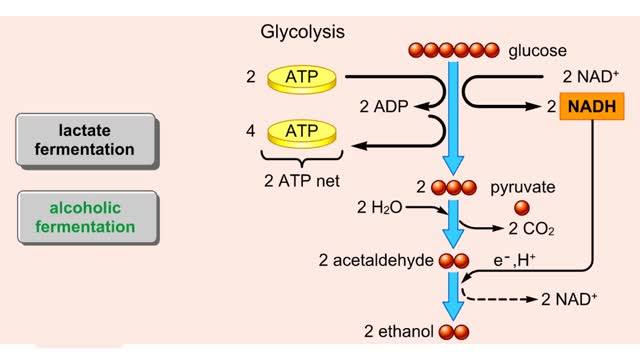
Both lactate fermentation and alcoholic fermentation begin with pyruvate formed by glycolysis. During lactate fermentation, pyruvate molecules accept electrons and hydrogen from NADH. This transfer regenerates NAD± and converts pyruvate to lactate. The net energy yield is the two ATP...
Energy inputs and release in glycolysis Animation
By: HWC, Views: 4867
Glycolysis breaks the six-carbon sugar glucose into two three-carbon molecules of pyruvate. The first steps of glycolysis require an energy input in the form of two phosphate-group transfers from ATP. These phosphorylations raise the energy level of glucose enough to allow the energy-releas...
Splitting of Sugar, Oxidation/ Reduction & ATP Generation
By: HWC, Views: 10738
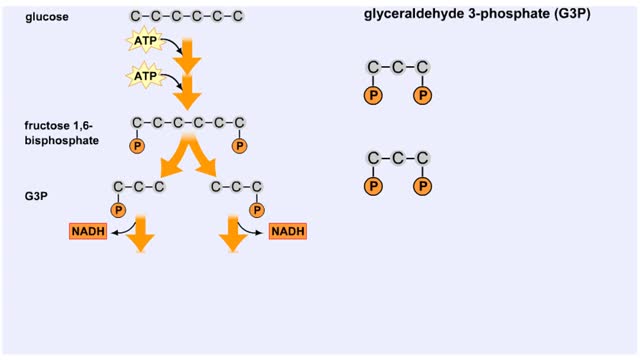
The next reaction shows us the meaning of "glycolysis" or the splitting of glucose. The fructose bisphosphate molecule is split into two molecules each containing 3 carbons as the backbone. FBP is split into two 3-carbon molecules called G3P, or glyceraldehyde 3-phosphate. Notice that the phos...
By: HWC, Views: 10505
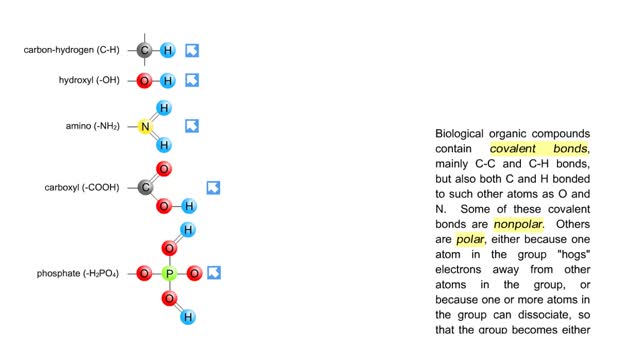
Biological organic compounds contain covalent bonds, mainly C-C and C-H bonds, but also both C and H bonded to such other atoms as O and N. Some of these covalent bonds are nonpolar. Others are polar, either because one atom in the group "hogs" electrons away from other atoms in the group, or...
By: HWC, Views: 11322
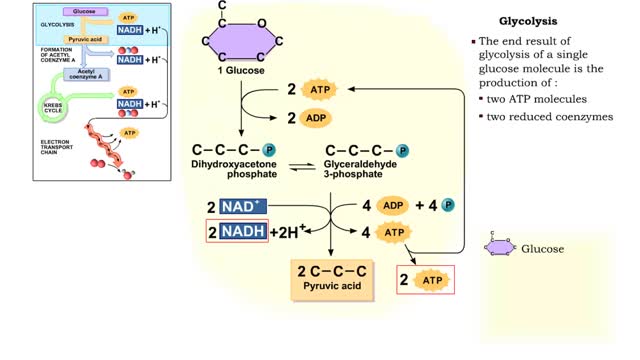
The first reactions involve a single 6-carbon glucose sugar undergoing phosphorylation using two ATP molecules and resulting in two 3-carbon compounds. • The rest of this pathway involves an oxidation reduction reaction, forming two reduced coenzymes, and generation of four ATP molecules. ...
By: HWC, Views: 10826
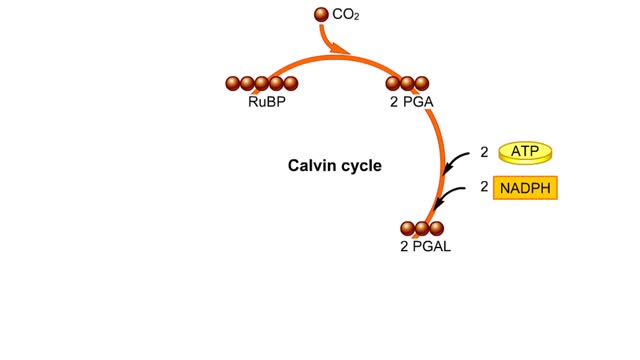
he light-independent reactions make sugars by way of a cyclic pathway called the Calvin cycle. The cycle begins when rubisco attaches a carbon from carbon dioxide to ribulose bisphosphate. The molecule that forms splits into two molecules of PGA. Each PGA gets a phosphate group from ATP a...
Advertisement