Bond types - Atomic structure and basis of bonds
By: HWC
Date Uploaded: 10/15/2019
Tags: homeworkclinic.com Homework Clinic HWC bond types covalent ionic bond hydrogen bond Electrons orbital carbohydrates nucleic acids الذرة الروابط أو الأواصر الكيميائية أنواع الروابط الكيميائية
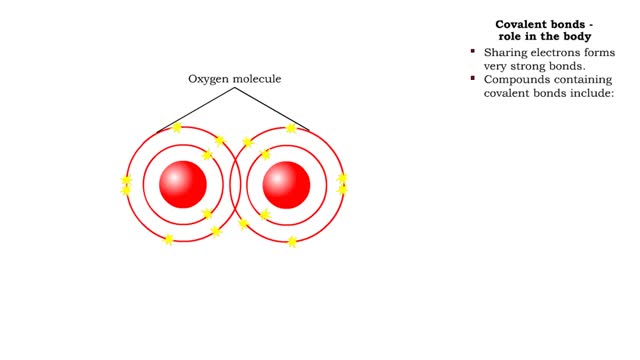
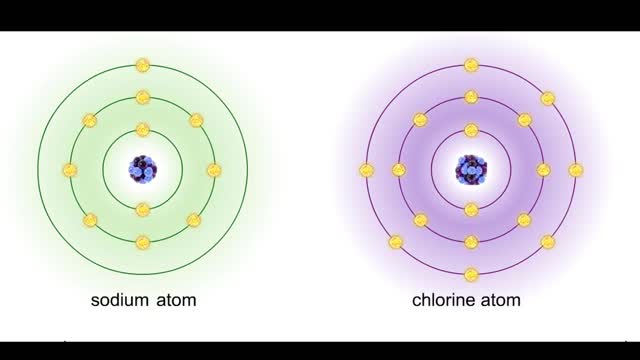
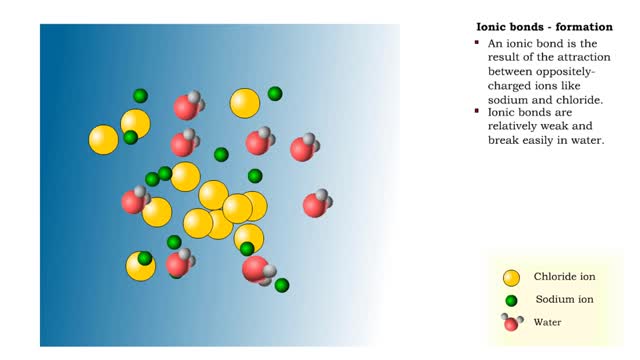
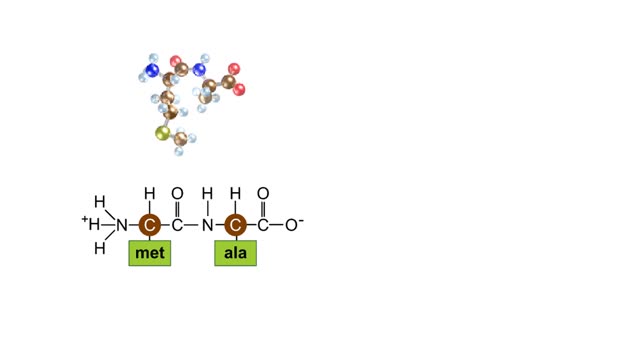
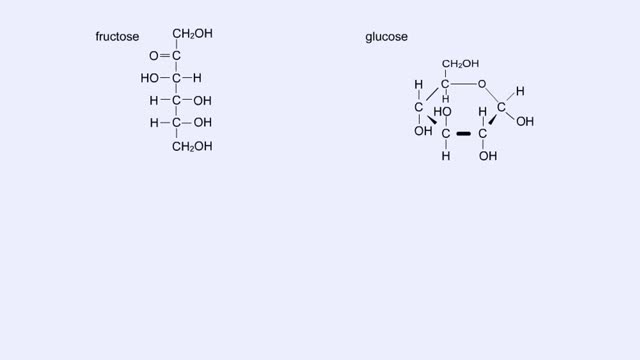
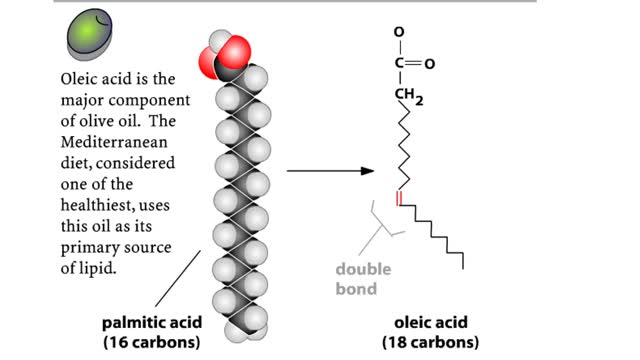
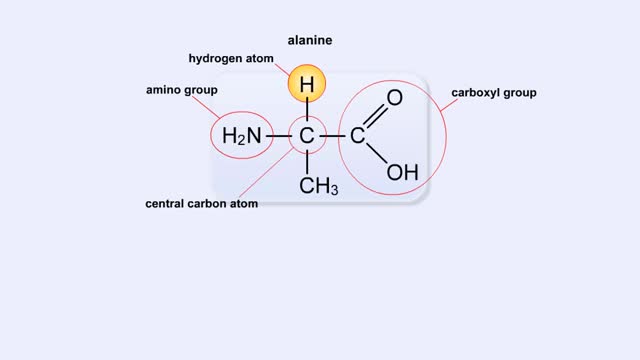
• Chemical bonds are fundamental to the structure and function of many types of molecules, such as proteins, carbohydrates, lipids, nucleic acids, gases, salts and water. ■ These molecules are composed of atoms that are held together by three different types of bonds. • The three types of bonds are covalent, ionic and hydrogen. ■ An atom consists of nucleus surrounded by electrons. ■ Electrons are found in orbitals around the nucleus. ■ Atoms are more stable when the outermost orbital is filled. ■ Atoms will shift electrons to fill up their outermost orbitals. ■ The basis of bonding is interaction of the outermost orbital electrons between atoms.
Add To
You must login to add videos to your playlists.
Advertisement





Comments
0 Comments total
Sign In to post comments.
No comments have been posted for this video yet.