Bond in biological molecules (Ionic, Covalent and Hydrogen bonds)/ How atoms bond?
By: HWC
Date Uploaded: 11/28/2021
Tags: How atoms bond? Bond in biological molecules Sodium atoms and chloride atoms outermost shell ionic bond crystal of table salt covalent bond nuclei nonpolar hydrogen nucleus molecule of ammonia a DNA molecule hydrogen bonds
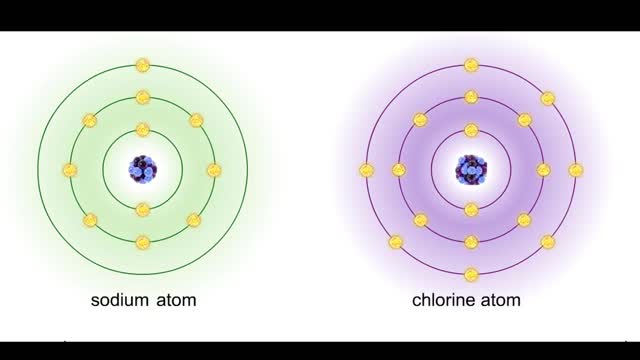
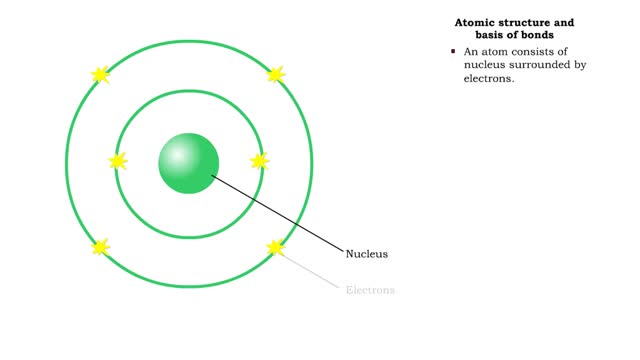
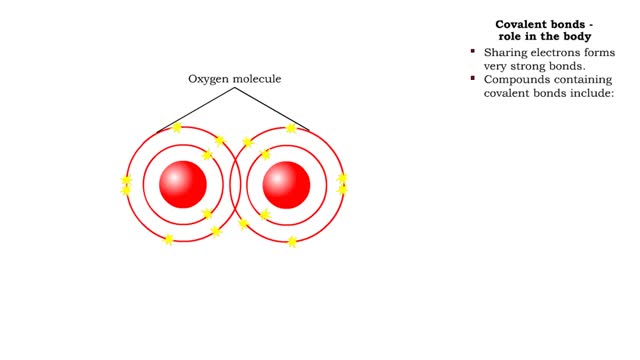
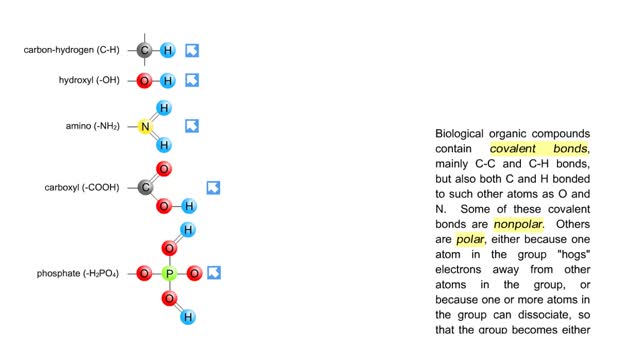
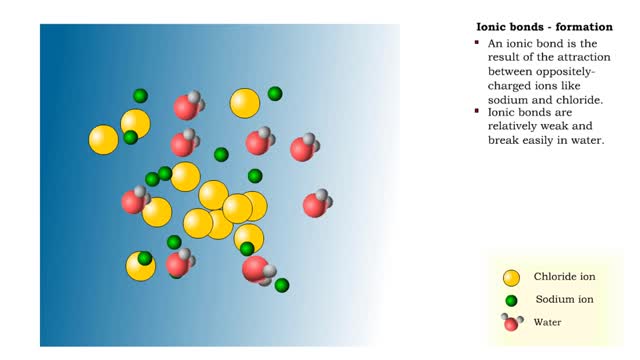
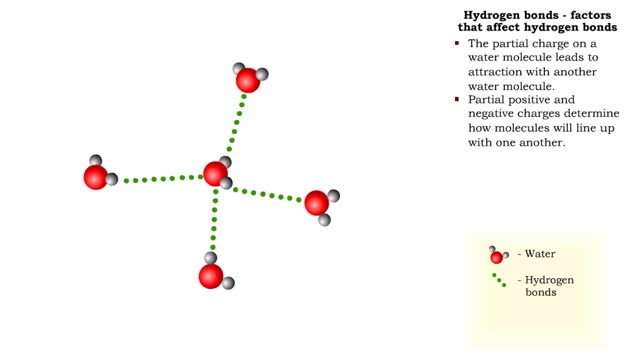
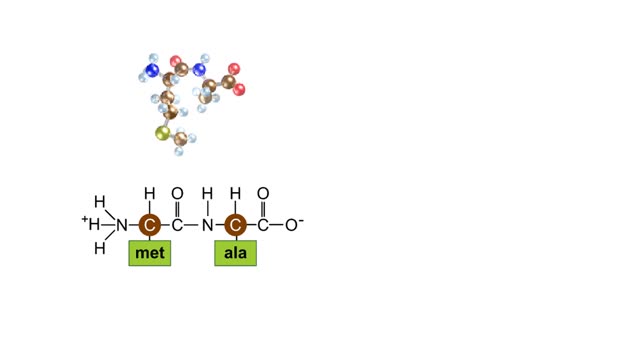
Sodium atoms and chloride atoms have unfilled orbitals in their outer shells. The lone electron in the outermost shell of a sodium atom can be pulled or knocked out. This ionizes the atom. It is now a positively charged sodium ion. A chlorine atom has an electron vacancy in its outer shell and can acquire another electron. This ionizes the atom to form a negatively charged chloride ion. The two oppositely charged ions attract each other. An association of two ions with opposing charges is known as an ionic bond. You can observe the outcome of ionic bonding in a portion of a crystal of table salt, or sodium chloride. In such crystals, sodium ions (Na') and chloride ions (C1) interact through ionic bonds. Two hydrogen atoms, each with an unpaired electron in the outer shell, can pair up to share a pair of electrons. This stabilizes the atoms and forms a single covalent bond. Both nuclei exert the same pull on the electrons, so they are shared equally and the bond is nonpolar. There is no charge difference between different parts of the molecule. An oxygen atom has two electron vacancies in its outer shell. Two oxygen atoms can share two pairs of electrons, forming a double covalent bond. Again, the electrons are distributed equally and the bond is nonpolar. Alternatively, an oxygen atom can share electron pairs with two hydrogen atoms. Each hydrogen atom shares a single pair of electrons with the oxygen—the molecule is held together by two single covalent bonds. An oxygen nucleus has more protons than a hydrogen nucleus, and so attracts the shared electrons more strongly. This unequal sharing gives the oxygen end of the atom a slight negative charge and the hydrogen end a slight positive charge. We say that water is held together by polar covalent bonds. The slight positive charge of a hydrogen atom in a water molecule can attract an atom with a slight negative charge, such as the nitrogen in a molecule of ammonia. This forms a hydrogen bond between the two atoms. Multiple hydrogen bonds can hold two large molecules together or stabilize the twists and folds of a single large protein. Hydrogen bonds join the two strands of a DNA molecule. Although hydrogen bonds are weaker than covalent bonds, extensive hydrogen bonding has a major role in the structure and behavior of DNA, water, proteins, and many other substances.
Add To
You must login to add videos to your playlists.
Advertisement



Comments
0 Comments total
Sign In to post comments.
No comments have been posted for this video yet.